'Click chemistry' synthesis of 1-(α-D-mannopyranosyl)-1,2,3-triazoles for inhibition of α-mannosidases
- PMID: 25658064
- PMCID: PMC4382718
- DOI: 10.1016/j.carres.2015.01.004
'Click chemistry' synthesis of 1-(α-D-mannopyranosyl)-1,2,3-triazoles for inhibition of α-mannosidases
Abstract
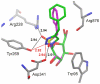
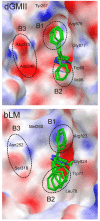
Three new triazole conjugates derived from d-mannose were synthesized and assayed in in vitro assays to investigate their ability to inhibit α-mannosidase enzymes from the glycoside hydrolase (GH) families 38 and 47. The triazole conjugates were more selective for a GH47 α-mannosidase (Aspergillus saitoi α1,2-mannosidase), showing inhibition at the micromolar level (IC50 values of 50-250 μM), and less potent towards GH38 mannosidases (IC50 values in the range of 0.5-6 mM towards jack bean α-mannosidase or Drosophila melanogaster lysosomal and Golgi α-mannosidases). The highest selectivity ratio [IC50(GH38)/IC50(GH47)] of 100 was exhibited by the phenyltriazole conjugate. To understand structure-activity properties of synthesized compounds, 3-D complexes of inhibitors with α-mannosidases were built using molecular docking calculations.
Keywords: 1-(d-Mannopyranosyl)-1,2,3-triazole; Click reaction; Glycoside hydrolase; Molecular docking; α-Mannosidase inhibitor.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Tornoe CW, Meldal M. Chem. Rev. 2008;108:2952–3015. - PubMed
-
- Kolb HC, Sharpless KB. Drug Discov. Today. 2003;8:1128–1137. - PubMed
-
- Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. - PubMed
-
- Lutz JF, Zarafshani Z. Adv. Drug Deliver. Rev. 2008;60:958–970. - PubMed
-
- Baskin JM, Bertozzi CR. Qsar Comb. Sci. 2007;26:1211–1219.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

