The anti-cancer potency and mechanism of a novel tumor-activated fused toxin, DLM
- PMID: 25658509
- PMCID: PMC4344633
- DOI: 10.3390/toxins7020423
The anti-cancer potency and mechanism of a novel tumor-activated fused toxin, DLM
Abstract
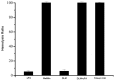
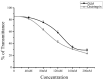
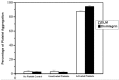
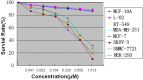
Melittin, which acts as a membrane-disrupting lytic peptide, is not only cytotoxic to tumors, but also vital to normal cells. Melittin had low toxicity when coupled with target peptides. Despite significant research development with the fused toxin, a new fused toxin is needed which has a cleavable linker such that the fused toxin can release melittin after protease cleavage on the tumor cell surface. We describe a novel fused toxin, composed of disintegrin, uPA (urokinase-type plasminogen activator)-cleavable linker, and melittin. Disintegrin is a single strand peptide (73 aa) isolated from Gloydius Ussuriensis venom. The RGD (Arg-Gly-Asp) site of disintegrin dominates its interaction with integrins on the surface of the tumor cells. uPA is over-expressed and plays an important role in tumor cell invasiveness and metastatic progression. The DLM (disintegrin-linker-melittin) linker is uPA-cleavable, enabling DLM to release melittin. We compared binding activity of our synthesized disintegrin with native disintegrin and report that DLM had less binding activity than the native form. uPA-cleavage was evaluated in vitro and the uPA-cleavable linker released melittin. Treating tumors expressing uPA with DLM enhanced tumor cell killing as well as reduced toxicity to erythrocytes and other non-cancerous normal cells. The mechanism behind DLM tumor cell killing was tested using a DNA ladder assay, fluorescent microscopy, flow cytometry, and transmission electron microscopy. Data revealed tumor cell necrosis as the mechanism of cell death, and the fused DLM toxin with an uPA-cleavable linker enhanced tumor selectivity and killing ability.
Figures








Similar articles
-
Expression and functional characterization of a recombinant targeted toxin with an uPA cleavable linker in Pichia pastoris.Protein Expr Purif. 2011 Apr;76(2):184-9. doi: 10.1016/j.pep.2010.12.001. Epub 2010 Dec 7. Protein Expr Purif. 2011. PMID: 21144903
-
Expression and anticancer activity analysis of recombinant human uPA1‑43-melittin.Int J Oncol. 2015 Feb;46(2):619-26. doi: 10.3892/ijo.2014.2750. Epub 2014 Nov 13. Int J Oncol. 2015. PMID: 25394558
-
Antigen binding and cytotoxic properties of a recombinant immunotoxin incorporating the lytic peptide, melittin.Immunotechnology. 1996 Sep;2(3):229-40. doi: 10.1016/s1380-2933(96)00055-3. Immunotechnology. 1996. PMID: 9373315
-
[Mechanism of tumor cell-induced extracellular matrix degradation--inhibition of cell-surface proteolytic activity might have a therapeutic effect on tumor cell invasion and metastasis].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):623-32. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808830 Review. Japanese.
-
Melittin: a lytic peptide with anticancer properties.Environ Toxicol Pharmacol. 2013 Sep;36(2):697-705. doi: 10.1016/j.etap.2013.06.009. Epub 2013 Jun 28. Environ Toxicol Pharmacol. 2013. PMID: 23892471 Review.
Cited by
-
Two Trichothecene Mycotoxins from Myrothecium roridum Induce Apoptosis of HepG-2 Cells via Caspase Activation and Disruption of Mitochondrial Membrane Potential.Molecules. 2016 Jun 17;21(6):781. doi: 10.3390/molecules21060781. Molecules. 2016. PMID: 27322225 Free PMC article.
-
Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy.Cancer Lett. 2017 Aug 28;402:16-31. doi: 10.1016/j.canlet.2017.05.010. Epub 2017 May 20. Cancer Lett. 2017. PMID: 28536009 Free PMC article. Review.
-
Recent advances in melittin-based nanoparticles for antitumor treatment: from mechanisms to targeted delivery strategies.J Nanobiotechnology. 2023 Nov 28;21(1):454. doi: 10.1186/s12951-023-02223-4. J Nanobiotechnology. 2023. PMID: 38017537 Free PMC article. Review.
-
Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects.Cell Mol Life Sci. 2017 Oct;74(20):3809-3825. doi: 10.1007/s00018-017-2604-z. Epub 2017 Aug 2. Cell Mol Life Sci. 2017. PMID: 28770291 Free PMC article. Review.
-
Use of Selected Carbon Nanoparticles as Melittin Carriers for MCF-7 and MDA-MB-231 Human Breast Cancer Cells.Materials (Basel). 2019 Dec 23;13(1):90. doi: 10.3390/ma13010090. Materials (Basel). 2019. PMID: 31878020 Free PMC article.
References
-
- Skeel R.T. Handbook of Cancer Chemotherapy. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2003.
-
- Takimoto C.H., Calvo E. Principles of oncologic pharmacotherapy. In: Pazdur R., Wagman L.D., Camphausen K.A., Hoskins W.J., editors. Cancer Management: A Multidisciplinary Approach. 11th ed. CMP United Business Media; Manhasset, NY, USA: 2009.
-
- Moertel C.G., Fleming T.R., Macdonald J.S., Haller D.G., Laurie J.A., Goodman P.J., Ungerleider J.S., Emerson W.A., Tormey D.C., Glick J.H., et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. - DOI - PubMed
-
- Soler M., González-Bártulos M., Soriano-Castell D., Ribas X., Costas M., Tebar F., Massaguer A., Feliu L., Planas M. Identification of BP16 as a non-toxic cell-penetrating peptide with highly efficient drug delivery properties. Org. Biomol. Chem. 2014;12:1652–1663. doi: 10.1039/c3ob42422g. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

