CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation
- PMID: 25658601
- PMCID: PMC4450086
- DOI: 10.1371/journal.ppat.1004663
CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation
Abstract
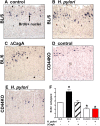
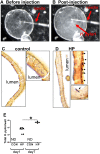
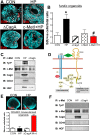
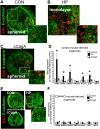
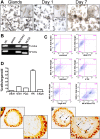
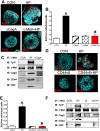
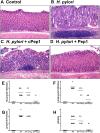
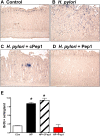
The cytotoxin-associated gene (Cag) pathogenicity island is a strain-specific constituent of Helicobacter pylori (H. pylori) that augments cancer risk. CagA translocates into the cytoplasm where it stimulates cell signaling through the interaction with tyrosine kinase c-Met receptor, leading cellular proliferation. Identified as a potential gastric stem cell marker, cluster-of-differentiation (CD) CD44 also acts as a co-receptor for c-Met, but whether it plays a functional role in H. pylori-induced epithelial proliferation is unknown. We tested the hypothesis that CD44 plays a functional role in H. pylori-induced epithelial cell proliferation. To assay changes in gastric epithelial cell proliferation in relation to the direct interaction with H. pylori, human- and mouse-derived gastric organoids were infected with the G27 H. pylori strain or a mutant G27 strain bearing cagA deletion (∆CagA::cat). Epithelial proliferation was quantified by EdU immunostaining. Phosphorylation of c-Met was analyzed by immunoprecipitation followed by Western blot analysis for expression of CD44 and CagA. H. pylori infection of both mouse- and human-derived gastric organoids induced epithelial proliferation that correlated with c-Met phosphorylation. CagA and CD44 co-immunoprecipitated with phosphorylated c-Met. The formation of this complex did not occur in organoids infected with ∆CagA::cat. Epithelial proliferation in response to H. pylori infection was lost in infected organoids derived from CD44-deficient mouse stomachs. Human-derived fundic gastric organoids exhibited an induction in proliferation when infected with H. pylori that was not seen in organoids pre-treated with a peptide inhibitor specific to CD44. In the well-established Mongolian gerbil model of gastric cancer, animals treated with CD44 peptide inhibitor Pep1, resulted in the inhibition of H. pylori-induced proliferation and associated atrophic gastritis. The current study reports a unique approach to study H. pylori interaction with the human gastric epithelium. Here, we show that CD44 plays a functional role in H. pylori-induced epithelial cell proliferation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311–1315. - PubMed
-
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M (1975) A model for gastric cancer epidemiology. Lancet 2: 58–60. - PubMed
-
- Kuipers EJ, Meuwissen SG (1996) Helicobacter pylori and gastric carcinogenesis. Scand J Gastroenterol 218: 103–105. - PubMed
-
- Kuipers EJ, Perez-Perez GI, Meuwissen SGM, Blaser MJ (1995) Helicobacter pylori and atrophic gastritis: Importance of the cagA status. J Natl Cancer Inst 87: 1777–1780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

