TnBP⁄Triton X-45 treatment of plasma for transfusion efficiently inactivates hepatitis C virus
- PMID: 25658612
- PMCID: PMC4320006
- DOI: 10.1371/journal.pone.0117800
TnBP⁄Triton X-45 treatment of plasma for transfusion efficiently inactivates hepatitis C virus
Abstract
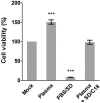
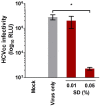
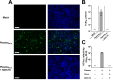
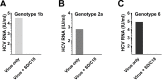
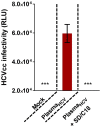
Risk of transmission of hepatitis C virus (HCV) by clinical plasma remains high in countries with a high prevalence of hepatitis C, justifying the implementation of viral inactivation treatments. In this study, we assessed the extent of inactivation of HCV during minipool solvent/detergent (SD; 1% TnBP / 1% Triton X-45) treatment of human plasma. Luciferase-tagged infectious cell culture-derived HCV (HCVcc) particles were used to spike human plasma prior to treatment by SD at 31 ± 0.5°C for 30 min. Samples were taken before and after SD treatment and filtered on a Sep-Pak Plus C18 cartridge to remove the SD agents. Risk of cytotoxicity was assessed by XTT cell viability assay. Viral infectivity was analyzed based on the luciferase signals, 50% tissue culture infectious dose viral titer, and immunofluorescence staining for HCV NS5A protein. Total protein, cholesterol, and triglyceride contents were determined before and after SD treatment and C18 cartridge filtration. Binding analysis, using patient-derived HCV clinical isolates, was also examined to validate the efficacy of the inactivation by SD. SD treatment effectively inactivated HCVcc within 30 min, as demonstrated by the baseline level of reporter signals, total loss of viral infectivity, and absence of viral protein NS5A. SD specifically targeted HCV particles to render them inactive, with essentially no effect on plasma protein content and hemostatic function. More importantly, the efficacy of the SD inactivation method was confirmed against various genotypes of patient-derived HCV clinical isolates and against HCVcc infection of primary human hepatocytes. Therefore, treatment by 1% TnBP / 1% Triton X-45 at 31°C is highly efficient to inactivate HCV in plasma for transfusion, showing its capacity to enhance the safety of therapeutic plasma products. We propose that the methodology used here to study HCV infectivity can be valuable in the validation of viral inactivation and removal processes of human plasma-derived products.
Conflict of interest statement
Figures
Similar articles
-
Impact of Triton X-100 on alpha 2-antiplasmin (SERPINF2) activity in solvent/detergent-treated plasma.Biologicals. 2007 Oct;35(4):349-53. doi: 10.1016/j.biologicals.2007.03.002. Epub 2007 Jul 26. Biologicals. 2007. PMID: 17656111
-
Dengue virus inactivation by minipool TnBP/Triton X-45 treatment of plasma and cryoprecipitate.Vox Sang. 2013 Jan;104(1):1-6. doi: 10.1111/j.1423-0410.2012.01621.x. Epub 2012 Jul 4. Vox Sang. 2013. PMID: 22758375
-
Thermal stability and inactivation of hepatitis C virus grown in cell culture.Virol J. 2010 Feb 18;7:40. doi: 10.1186/1743-422X-7-40. Virol J. 2010. PMID: 20167059 Free PMC article.
-
Viral safety of solvent-detergent treated blood products.Dev Biol Stand. 1993;81:147-61. Dev Biol Stand. 1993. PMID: 8174797 Review.
-
[Virus inactivated plasma].Infusionsther Transfusionsmed. 1994 Aug;21 Suppl 1:73-6. Infusionsther Transfusionsmed. 1994. PMID: 8000259 Review. German.
Cited by
-
Validation of Viral Inactivation Protocols for Therapeutic Blood Products against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2).Viruses. 2022 Oct 31;14(11):2419. doi: 10.3390/v14112419. Viruses. 2022. PMID: 36366517 Free PMC article.
-
Solvent/Detergent Virally Inactivated Serum Eye Drops Restore Healthy Ocular Epithelium in a Rabbit Model of Dry-Eye Syndrome.PLoS One. 2016 Apr 21;11(4):e0153573. doi: 10.1371/journal.pone.0153573. eCollection 2016. PLoS One. 2016. PMID: 27100624 Free PMC article.
-
Contemporary resuscitation of hemorrhagic shock: What will the future hold?Am J Surg. 2020 Sep;220(3):580-588. doi: 10.1016/j.amjsurg.2020.05.008. Epub 2020 May 11. Am J Surg. 2020. PMID: 32409009 Free PMC article. Review.
-
Implementation of Plasma Fractionation in Biological Medicines Production.Iran J Biotechnol. 2016 Dec;14(4):213-220. doi: 10.15171/ijb.1401. Iran J Biotechnol. 2016. PMID: 28959338 Free PMC article. Review.
-
Towards pathogen inactivation of red blood cells and whole blood targeting viral DNA/RNA: design, technologies, and future prospects for developing countries.Blood Transfus. 2017 Oct;15(6):512-521. doi: 10.2450/2017.0344-16. Epub 2017 Apr 13. Blood Transfus. 2017. PMID: 28488960 Free PMC article. Review.
References
-
- O'shaughnessy D, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, et al. (2004) Guidelines for the use of fresh‐frozen plasma, cryoprecipitate and cryosupernatant. British journal of haematology 126: 11–28. - PubMed
-
- Kakkar N, Kaur R, Dhanoa J (2004) Improvement in fresh frozen plasma transfusion practice: results of an outcome audit. Transfusion Medicine 14: 231–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

