Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice
- PMID: 25658614
- PMCID: PMC4321765
- DOI: 10.1097/CJI.0000000000000064
Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice
Abstract
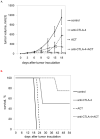
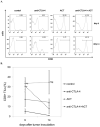
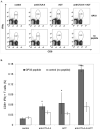
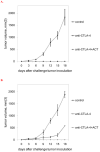
Immunotherapeutic approaches to the treatment of advanced melanoma have relied on strategies that augment the responsiveness of endogenous tumor-specific T-cell populations [eg, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade-mediated checkpoint inhibition] or introduce exogenously prepared tumor-specific T-cell populations [eg, adoptive cell transfer (ACT)]. Although both approaches have shown considerable promise, response rates to these therapies remain suboptimal. We hypothesized that a combinatorial approach to immunotherapy using both CTLA-4 blockade and nonlymphodepletional ACT could offer additive therapeutic benefit. C57BL/6 mice were inoculated with syngeneic B16F10 melanoma tumors transfected to express low levels of the lymphocytic choriomeningitis virus peptide GP33 (B16GP33), and treated with no immunotherapy, CTLA-4 blockade, ACT, or combination immunotherapy of CTLA-4 blockade with ACT. Combination immunotherapy resulted in optimal control of B16GP33 melanoma tumors. Combination immunotherapy promoted a stronger local immune response reflected by enhanced tumor-infiltrating lymphocyte populations, and a stronger systemic immune responses reflected by more potent tumor antigen-specific T-cell activity in splenocytes. In addition, whereas both CTLA-4 blockade and combination immunotherapy were able to promote long-term immunity against B16GP33 tumors, only combination immunotherapy was capable of promoting immunity against parental B16F10 tumors as well. Our findings suggest that a combinatorial approach using CTLA-4 blockade with nonlymphodepletional ACT may promote additive endogenous and exogenous T-cell activities that enable greater therapeutic efficacy in the treatment of melanoma.
Figures


References
-
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. - PubMed
-
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. - PMC - PubMed
-
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. - PMC - PubMed
-
- Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–261. - PubMed
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanan JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg F, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

