Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium Antagonist
- PMID: 25658622
- PMCID: PMC4450071
- DOI: 10.1371/journal.ppat.1004631
Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium Antagonist
Abstract
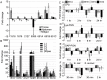
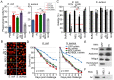
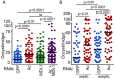
Actin is a highly versatile, abundant, and conserved protein, with functions in a variety of intracellular processes. Here, we describe a novel role for insect cytoplasmic actin as an extracellular pathogen recognition factor that mediates antibacterial defense. Insect actins are secreted from cells upon immune challenge through an exosome-independent pathway. Anopheles gambiae actin interacts with the extracellular MD2-like immune factor AgMDL1, and binds to the surfaces of bacteria, mediating their phagocytosis and direct killing. Globular and filamentous actins display distinct functions as extracellular immune factors, and mosquito actin is a Plasmodium infection antagonist.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

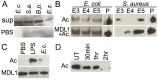
Similar articles
-
Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway.Cell Host Microbe. 2008 Jun 12;3(6):364-74. doi: 10.1016/j.chom.2008.05.007. Cell Host Microbe. 2008. PMID: 18541213 Review.
-
NF-κB-Like Signaling Pathway REL2 in Immune Defenses of the Malaria Vector Anopheles gambiae.Front Cell Infect Microbiol. 2017 Jun 21;7:258. doi: 10.3389/fcimb.2017.00258. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680852 Free PMC article. Review.
-
The Peptidoglycan Recognition Proteins PGRPLA and PGRPLB Regulate Anopheles Immunity to Bacteria and Affect Infection by Plasmodium.J Innate Immun. 2017;9(4):333-342. doi: 10.1159/000452797. Epub 2017 May 12. J Innate Immun. 2017. PMID: 28494453 Free PMC article.
-
Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action.PLoS Pathog. 2012;8(6):e1002737. doi: 10.1371/journal.ppat.1002737. Epub 2012 Jun 7. PLoS Pathog. 2012. PMID: 22685401 Free PMC article.
-
Molecular Profiling of Phagocytic Immune Cells in Anopheles gambiae Reveals Integral Roles for Hemocytes in Mosquito Innate Immunity.Mol Cell Proteomics. 2016 Nov;15(11):3373-3387. doi: 10.1074/mcp.M116.060723. Epub 2016 Sep 13. Mol Cell Proteomics. 2016. PMID: 27624304 Free PMC article.
Cited by
-
Targeting the mosquito prefoldin-chaperonin complex blocks Plasmodium transmission.Nat Microbiol. 2025 Apr;10(4):841-854. doi: 10.1038/s41564-025-01947-3. Epub 2025 Mar 6. Nat Microbiol. 2025. PMID: 40050397
-
Proteomic analysis unveils host-parasite interactions in Aedes togoi infected with Dirofilaria immitis and Brugia pahangi.PLoS One. 2025 Jul 9;20(7):e0326693. doi: 10.1371/journal.pone.0326693. eCollection 2025. PLoS One. 2025. PMID: 40632758 Free PMC article.
-
Arthropod transcriptional activator protein-1 (AP-1) aids tick-rickettsial pathogen survival in the cold.Sci Rep. 2018 Jul 30;8(1):11409. doi: 10.1038/s41598-018-29654-6. Sci Rep. 2018. PMID: 30061607 Free PMC article.
-
Reference gene identification and evaluation for accurate qPCR normalization in Phenacoccus manihoti Matile-Ferrero (Hemiptera: Pseudococcidae).Mol Biol Rep. 2025 Aug 21;52(1):838. doi: 10.1007/s11033-025-10922-4. Mol Biol Rep. 2025. PMID: 40839229 No abstract available.
-
Toll9 from Bombyx mori functions as a pattern recognition receptor that shares features with Toll-like receptor 4 from mammals.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2103021118. doi: 10.1073/pnas.2103021118. Proc Natl Acad Sci U S A. 2021. PMID: 33963082 Free PMC article.
References
-
- May RC, Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114: 1061–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

