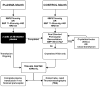
Taking the Blood Bank to the Field: The Design and Rationale of the Prehospital Air Medical Plasma (PAMPer) Trial
- PMID: 25658881
- PMCID: PMC4618798
- DOI: 10.3109/10903127.2014.995851
Taking the Blood Bank to the Field: The Design and Rationale of the Prehospital Air Medical Plasma (PAMPer) Trial
Abstract
Hemorrhage and trauma induced coagulopathy remain major drivers of early preventable mortality in military and civilian trauma. Interest in the use of prehospital plasma in hemorrhaging patients as a primary resuscitation agent has grown recently. Trauma center-based damage control resuscitation using early and aggressive plasma transfusion has consistently demonstrated improved outcomes in hemorrhaging patients. Additionally, plasma has been shown to have several favorable immunomodulatory effects. Preliminary evidence with prehospital plasma transfusion has demonstrated feasibility and improved short-term outcomes. Applying state-of-the-art resuscitation strategies to the civilian prehospital arena is compelling. We describe here the rationale, design, and challenges of the Prehospital Air Medical Plasma (PAMPer) trial. The primary objective is to determine the effect of prehospital plasma transfusion during air medical transport on 30-day mortality in patients at risk for traumatic hemorrhage. This study is a multicenter cluster randomized clinical trial. The trial will enroll trauma patients with profound hypotension (SBP ≤ 70 mmHg) or hypotension (SBP 71-90 mmHg) and tachycardia (HR ≥ 108 bpm) from six level I trauma center air medical transport programs. The trial will also explore the effects of prehospital plasma transfusion on the coagulation and inflammatory response following injury. The trial will be conducted under exception for informed consent for emergency research with an investigational new drug approval from the U.S. Food and Drug Administration utilizing a multipronged community consultation process. It is one of three ongoing Department of Defense-funded trials aimed at expanding our understanding of the optimal therapeutic approaches to coagulopathy in the hemorrhaging trauma patient.
Keywords: clinical trial; helicopter.; plasma; prehospital; randomized; trauma.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. - PubMed
-
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. - PubMed
-
- Rahbar E, Fox EE, del Junco DJ, Harvin JA, Holcomb JB, Wade CE, Schreiber MA, Rahbar MH, Bulger EM, Phelan HA, Brasel KJ, Alarcon LH, Myers JG, Cohen MJ, Muskat P, Cotton BA. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75:S16–S23. - PMC - PubMed
-
- Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, Wolf PL. The epidemiology of traumatic death: a population-based analysis. Arch Surg. 1993;128:571–5. - PubMed
-
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases