Asymmetry of the budding yeast Tem1 GTPase at spindle poles is required for spindle positioning but not for mitotic exit
- PMID: 25658911
- PMCID: PMC4450052
- DOI: 10.1371/journal.pgen.1004938
Asymmetry of the budding yeast Tem1 GTPase at spindle poles is required for spindle positioning but not for mitotic exit
Abstract
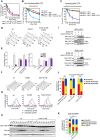
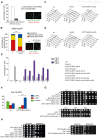
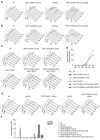
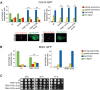
The asymmetrically dividing yeast S. cerevisiae assembles a bipolar spindle well after establishing the future site of cell division (i.e., the bud neck) and the division axis (i.e., the mother-bud axis). A surveillance mechanism called spindle position checkpoint (SPOC) delays mitotic exit and cytokinesis until the spindle is properly positioned relative to the mother-bud axis, thereby ensuring the correct ploidy of the progeny. SPOC relies on the heterodimeric GTPase-activating protein Bub2/Bfa1 that inhibits the small GTPase Tem1, in turn essential for activating the mitotic exit network (MEN) kinase cascade and cytokinesis. The Bub2/Bfa1 GAP and the Tem1 GTPase form a complex at spindle poles that undergoes a remarkable asymmetry during mitosis when the spindle is properly positioned, with the complex accumulating on the bud-directed old spindle pole. In contrast, the complex remains symmetrically localized on both poles of misaligned spindles. The mechanism driving asymmetry of Bub2/Bfa1/Tem1 in mitosis is unclear. Furthermore, whether asymmetry is involved in timely mitotic exit is controversial. We investigated the mechanism by which the GAP Bub2/Bfa1 controls GTP hydrolysis on Tem1 and generated a series of mutants leading to constitutive Tem1 activation. These mutants are SPOC-defective and invariably lead to symmetrical localization of Bub2/Bfa1/Tem1 at spindle poles, indicating that GTP hydrolysis is essential for asymmetry. Constitutive tethering of Bub2 or Bfa1 to both spindle poles impairs SPOC response but does not impair mitotic exit. Rather, it facilitates mitotic exit of MEN mutants, likely by increasing the residence time of Tem1 at spindle poles where it gets active. Surprisingly, all mutant or chimeric proteins leading to symmetrical localization of Bub2/Bfa1/Tem1 lead to increased symmetry at spindle poles of the Kar9 protein that mediates spindle positioning and cause spindle misalignment. Thus, asymmetry of the Bub2/Bfa1/Tem1 complex is crucial to control Kar9 distribution and spindle positioning during mitosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Coupling spindle position with mitotic exit in budding yeast: The multifaceted role of the small GTPase Tem1.Small GTPases. 2015 Oct 2;6(4):196-201. doi: 10.1080/21541248.2015.1109023. Small GTPases. 2015. PMID: 26507466 Free PMC article. Review.
-
Disappearance of the budding yeast Bub2-Bfa1 complex from the mother-bound spindle pole contributes to mitotic exit.J Cell Biol. 2006 Jan 30;172(3):335-46. doi: 10.1083/jcb.200507162. J Cell Biol. 2006. PMID: 16449187 Free PMC article.
-
The N-Terminal Domain of Bfa1 Coordinates Mitotic Exit Independent of GAP Activity in Saccharomyces cerevisiae.Cells. 2022 Jul 12;11(14):2179. doi: 10.3390/cells11142179. Cells. 2022. PMID: 35883622 Free PMC article.
-
A dynamical model of the spindle position checkpoint.Mol Syst Biol. 2012 May 8;8:582. doi: 10.1038/msb.2012.15. Mol Syst Biol. 2012. PMID: 22580890 Free PMC article.
-
The mother-bud neck as a signaling platform for the coordination between spindle position and cytokinesis in budding yeast.Biol Chem. 2011 Aug;392(8-9):805-12. doi: 10.1515/BC.2011.090. Biol Chem. 2011. PMID: 21824008 Review.
Cited by
-
Spindle pole bodies function as signal amplifiers in the Mitotic Exit Network.Mol Biol Cell. 2020 Apr 15;31(9):906-916. doi: 10.1091/mbc.E19-10-0584. Epub 2020 Feb 19. Mol Biol Cell. 2020. PMID: 32074005 Free PMC article.
-
Nuclear movement in fungi.Semin Cell Dev Biol. 2018 Oct;82:3-16. doi: 10.1016/j.semcdb.2017.10.024. Epub 2017 Dec 11. Semin Cell Dev Biol. 2018. PMID: 29241689 Free PMC article. Review.
-
Unifying the mechanism of mitotic exit control in a spatiotemporal logical model.PLoS Biol. 2020 Nov 12;18(11):e3000917. doi: 10.1371/journal.pbio.3000917. eCollection 2020 Nov. PLoS Biol. 2020. PMID: 33180788 Free PMC article.
-
SIN-Like Pathway Kinases Regulate the End of Mitosis in the Methylotrophic Yeast Ogataea polymorpha.Cells. 2022 Apr 30;11(9):1519. doi: 10.3390/cells11091519. Cells. 2022. PMID: 35563825 Free PMC article.
-
Budding yeast Wee1 distinguishes spindle pole bodies to guide their pattern of age-dependent segregation.Nat Cell Biol. 2017 Aug;19(8):941-951. doi: 10.1038/ncb3576. Epub 2017 Jul 17. Nat Cell Biol. 2017. PMID: 28714971
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

