In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria
- PMID: 25658925
- PMCID: PMC4450059
- DOI: 10.1371/journal.ppat.1004598
In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria
Abstract
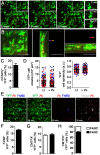
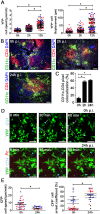
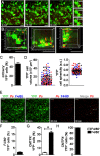
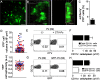
Dendritic cells (DCs) are phagocytes that are highly specialized for antigen presentation. Heterogeneous populations of macrophages and DCs form a phagocyte network inside the red pulp (RP) of the spleen, which is a major site for the control of blood-borne infections such as malaria. However, the dynamics of splenic DCs during Plasmodium infections are poorly understood, limiting our knowledge regarding their protective role in malaria. Here, we used in vivo experimental approaches that enabled us to deplete or visualize DCs in order to clarify these issues. To elucidate the roles of DCs and marginal zone macrophages in the protection against blood-stage malaria, we infected DTx (diphtheria toxin)-treated C57BL/6.CD11c-DTR mice, as well as C57BL/6 mice treated with low doses of clodronate liposomes (ClLip), with Plasmodium chabaudi AS (Pc) parasites. The first evidence suggesting that DCs could contribute directly to parasite clearance was an early effect of the DTx treatment, but not of the ClLip treatment, in parasitemia control. DCs were also required for CD4+ T cell responses during infection. The phagocytosis of infected red blood cells (iRBCs) by splenic DCs was analyzed by confocal intravital microscopy, as well as by flow cytometry and immunofluorescence, at three distinct phases of Pc malaria: at the first encounter, at pre-crisis concomitant with parasitemia growth and at crisis when the parasitemia decline coincides with spleen closure. In vivo and ex vivo imaging of the spleen revealed that DCs actively phagocytize iRBCs and interact with CD4+ T cells both in T cell-rich areas and in the RP. Subcapsular RP DCs were highly efficient in the recognition and capture of iRBCs during pre-crisis, while complete DC maturation was only achieved during crisis. These findings indicate that, beyond their classical role in antigen presentation, DCs also contribute to the direct elimination of iRBCs during acute Plasmodium infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Steiniger B, Barth P (2000) Microanatomy and function of the spleen. Adv Anat Embryol Cell Biol 151: III–IX, 1–101. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

