Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease
- PMID: 25659076
- PMCID: PMC4319910
- DOI: 10.1371/journal.pone.0113482
Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease
Abstract
Objectives: The extent and relevance of altered bone metabolism for statural growth in children with chronic kidney disease is controversial. We analyzed the impact of renal dysfunction and recombinant growth hormone therapy on a panel of serum markers of bone metabolism in a large pediatric chronic kidney disease cohort.
Methods: Bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase 5b (TRAP5b), sclerostin and C-terminal FGF-23 (cFGF23) normalized for age and sex were analyzed in 556 children aged 6-18 years with an estimated glomerular filtration rate (eGFR) of 10-60 ml/min/1.73 m2. 41 children receiving recombinant growth hormone therapy were compared to an untreated matched control group.
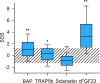
Results: Standardized levels of BAP, TRAP5b and cFGF-23 were increased whereas sclerostin was reduced. BAP was correlated positively and cFGF-23 inversely with eGFR. Intact serum parathormone was an independent positive predictor of BAP and TRAP5b and negatively associated with sclerostin. BAP and TRAP5B were negatively affected by increased C-reactive protein levels. In children receiving recombinant growth hormone, BAP was higher and TRAP5b lower than in untreated controls. Sclerostin levels were in the normal range and higher than in untreated controls. Serum sclerostin and cFGF-23 independently predicted height standard deviation score, and BAP and TRAP5b the prospective change in height standard deviation score.
Conclusion: Markers of bone metabolism indicate a high-bone turnover state in children with chronic kidney disease. Growth hormone induces an osteoanabolic pattern and normalizes osteocyte activity. The osteocyte markers cFGF23 and sclerostin are associated with standardized height, and the markers of bone turnover predict height velocity.
Conflict of interest statement
Figures

References
-
- Wesseling-Perry K, Salusky IB (2013) Chronic kidney disease: mineral and bone disorder in children. YSNEP 33: 169–179. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

