IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells
- PMID: 25659095
- PMCID: PMC4450060
- DOI: 10.1371/journal.ppat.1004607
IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells
Abstract
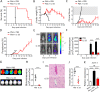
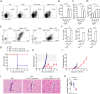
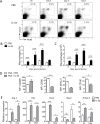
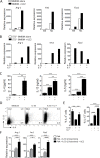
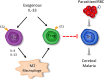
Cerebral malaria (CM) is a complex parasitic disease caused by Plasmodium sp. Failure to establish an appropriate balance between pro- and anti-inflammatory immune responses is believed to contribute to the development of cerebral pathology. Using the blood-stage PbA (Plasmodium berghei ANKA) model of infection, we show here that administration of the pro-Th2 cytokine, IL-33, prevents the development of experimental cerebral malaria (ECM) in C57BL/6 mice and reduces the production of inflammatory mediators IFN-γ, IL-12 and TNF-α. IL-33 drives the expansion of type-2 innate lymphoid cells (ILC2) that produce Type-2 cytokines (IL-4, IL-5 and IL-13), leading to the polarization of the anti-inflammatory M2 macrophages, which in turn expand Foxp3 regulatory T cells (Tregs). PbA-infected mice adoptively transferred with ILC2 have elevated frequency of M2 and Tregs and are protected from ECM. Importantly, IL-33-treated mice deleted of Tregs (DEREG mice) are no longer able to resist ECM. Our data therefore provide evidence that IL-33 can prevent the development of ECM by orchestrating a protective immune response via ILC2, M2 macrophages and Tregs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization (2012) World Malaria Report 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

