The role of phosphoinositide 3-kinases in neutrophil migration in 3D collagen gels
- PMID: 25659107
- PMCID: PMC4320071
- DOI: 10.1371/journal.pone.0116250
The role of phosphoinositide 3-kinases in neutrophil migration in 3D collagen gels
Erratum in
-
Correction: The Role of Phosphoinositide 3-Kinases in Neutrophil Migration in 3D Collagen Gels.PLoS One. 2015 Jul 31;10(7):e0134924. doi: 10.1371/journal.pone.0134924. eCollection 2015. PLoS One. 2015. PMID: 26230259 Free PMC article.
Abstract
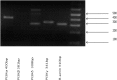
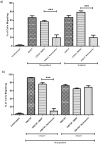
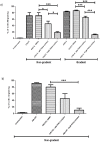
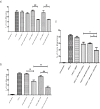
The entry of neutrophils into tissue has been well characterised; however the fate of these cells once inside the tissue microenvironment is not fully understood. A variety of signal transduction pathways including those involving class I PI3 Kinases have been suggested to be involved in neutrophil migration. This study aims to determine the involvement of PI3 Kinases in chemokinetic and chemotactic neutrophil migration in response to CXCL8 and GM-CSF in a three-dimensional collagen gel, as a model of tissue. Using a three-dimensional collagen assay chemokinetic and chemotactic migration induced by CXCL8 was inhibited with the pan PI3 Kinase inhibitor wortmannin. Analysis of the specific Class I PI3 Kinase catalytic isoforms alpha, delta and gamma using the inhibitors PIK-75, PIK-294 and AS-605240 respectively indicated differential roles in CXCL8-induced neutrophil migration. PIK-294 inhibited both chemokinetic and chemotactic CXCL8-induced migration. AS-605240 markedly reduced CXCL8 induced chemokinetic migration but had no effect on CXCL8 induced chemotactic migration. In contrast PIK-75 inhibited chemotactic migration but not chemokinetic migration. At optimal concentrations of GM-CSF the inhibitors had no effect on the percentage of neutrophil migration in comparison to the control however at suboptimal concentrations wortmannin, AS-605240 and PIK-294 inhibited chemokinesis. This study suggests that PI3 Kinase is necessary for CXCL8 induced migration in a 3D tissue environment but that chemokinetic and chemotactic migration may be controlled by different isoforms with gamma shown to be important in chemokinesis and alpha important in chemotaxis. Neutrophil migration in response to suboptimal concentrations of GM-CSF is dependent on PI3 Kinase, particularly the gamma and delta catalytic isoforms.
Conflict of interest statement
Figures



References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews.Immunology 7: 678–689. - PubMed
-
- Knall C, Worthen GS, Johnson GL (1997) Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl. Acad. Sci. U. S. A. 94: 3052–3057. - PMC - PubMed
-
- Knall C, Young S, Nick JA, Buhl AM, Worthen GS, et al. (1996) Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem. 271: 2832–2838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

