High prevalence of the K65R mutation in HIV-1 subtype C infected patients failing tenofovir-based first-line regimens in South Africa
- PMID: 25659108
- PMCID: PMC4320083
- DOI: 10.1371/journal.pone.0118145
High prevalence of the K65R mutation in HIV-1 subtype C infected patients failing tenofovir-based first-line regimens in South Africa
Abstract
Background: Tenofovir (TDF) has replaced stavudine (d4T) as the preferred nucleoside reverse transcriptase inhibitor (NRTI) in first-line regimens in South Africa, but limited information is available on the resistance patterns that develop after the introduction of TDF. This study investigated the antiretroviral drug resistance patterns in South African HIV-1 subtype C-infected patients failing stavudine- (d4T) and tenofovir- (TDF) based first-line regimens and assess the suitability of TDF as the preferred first-line nucleotide reverse transcriptase inhibitor (NRTI).
Methods: Resistance patterns of HIV-1 from 160 adult patients virologically failing TDF- (n = 80) and d4T- (n = 80) based first-line regimens were retrospectively analyzed. The pol gene was sequenced using an in-house protocol and mutations were analysed using the IAS-USA 2014 Drug Resistance Mutation list.
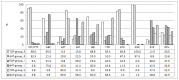
Results: Compared to d4T-exposed patients (n = 7), patients failing on a TDF-containing regimen (n = 43) were almost 5 times more likely to present with a K65R mutation (aRR 4.86 95% CI 2.29 - 10.34). Y115F was absent in the d4T group, and detected in 13.8% (n = 11) of TDF-exposed patients, p = 0.0007. Virus from 9 of the 11 patients (82.0%) who developed the Y115F mutation also developed K65R. Intermediate or high-level resistance to most NRTIs was common in the TDF-treatment group, but these patients twice more likely to remain susceptible to AZT as compared to those exposed to d4T (aRR 2.09 95% CI 1.13 - 3.90).
Conclusion: The frequency of the TDF induced K65R mutation was higher in our setting compared to non-subtype C dominated countries. However, despite the higher frequency of cross-resistance to NRTIs, most patients remained susceptible to AZT, which is reflected in the South African treatment guidelines that recommend AZT as an essential component of second-line regimens.
Conflict of interest statement
Figures


References
-
- WHO (2013) Global Update on HIV treatment 2013: results, impact and opportunities. World Health Organization.
-
- Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, et al. (2002) Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr 30: 105–110. - PubMed
-
- Novitsky V, Wester CW, DeGruttola V, Bussmann H, Gaseitsiwe S, et al. (2007) The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses 23: 868–878. - PubMed
-
- (2004) South African Antiretroviral Treatment Guidelines. In: NDoH, editor.
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

