Aberrant DNA damage response pathways may predict the outcome of platinum chemotherapy in ovarian cancer
- PMID: 25659114
- PMCID: PMC4320060
- DOI: 10.1371/journal.pone.0117654
Aberrant DNA damage response pathways may predict the outcome of platinum chemotherapy in ovarian cancer
Erratum in
-
Correction: Aberrant DNA Damage Response Pathways May Predict the Outcome of Platinum Chemotherapy in Ovarian Cancer.PLoS One. 2021 Aug 5;16(8):e0256051. doi: 10.1371/journal.pone.0256051. eCollection 2021. PLoS One. 2021. PMID: 34352021 Free PMC article.
Abstract
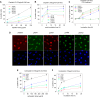
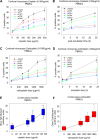
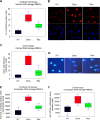
Ovarian carcinoma (OC) is the most lethal gynecological malignancy. Despite the advances in the treatment of OC with combinatorial regimens, including surgery and platinum-based chemotherapy, patients generally exhibit poor prognosis due to high chemotherapy resistance. Herein, we tested the hypothesis that DNA damage response (DDR) pathways are involved in resistance of OC patients to platinum chemotherapy. Selected DDR signals were evaluated in two human ovarian carcinoma cell lines, one sensitive (A2780) and one resistant (A2780/C30) to platinum treatment as well as in peripheral blood mononuclear cells (PBMCs) from OC patients, sensitive (n = 7) or resistant (n = 4) to subsequent chemotherapy. PBMCs from healthy volunteers (n = 9) were studied in parallel. DNA damage was evaluated by immunofluorescence γH2AX staining and comet assay. Higher levels of intrinsic DNA damage were found in A2780 than in A2780/C30 cells. Moreover, the intrinsic DNA damage levels were significantly higher in OC patients relative to healthy volunteers, as well as in platinum-sensitive patients relative to platinum-resistant ones (all P<0.05). Following carboplatin treatment, A2780 cells showed lower DNA repair efficiency than A2780/C30 cells. Also, following carboplatin treatment of PBMCs ex vivo, the DNA repair efficiency was significantly higher in healthy volunteers than in platinum-resistant patients and lowest in platinum-sensitive ones (t1/2 for loss of γH2AX foci: 2.7±0.5h, 8.8±1.9h and 15.4±3.2h, respectively; using comet assay, t1/2 of platinum-induced damage repair: 4.8±1.4h, 12.9±1.9h and 21.4±2.6h, respectively; all P<0.03). Additionally, the carboplatin-induced apoptosis rate was higher in A2780 than in A2780/C30 cells. In PBMCs, apoptosis rates were inversely correlated with DNA repair efficiencies of these cells, being significantly higher in platinum-sensitive than in platinum-resistant patients and lowest in healthy volunteers (all P<0.05). We conclude that perturbations of DNA repair pathways as measured in PBMCs from OC patients correlate with the drug sensitivity of these cells and reflect the individualized response to platinum-based chemotherapy.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

