Lewis acid-induced change from four- to two-electron reduction of dioxygen catalyzed by copper complexes using scandium triflate
- PMID: 25659416
- PMCID: PMC4630010
- DOI: 10.1021/ja512584r
Lewis acid-induced change from four- to two-electron reduction of dioxygen catalyzed by copper complexes using scandium triflate
Abstract
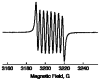
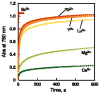
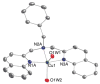
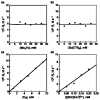
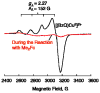
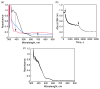
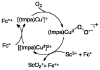
Mononuclear copper complexes, [(tmpa)Cu(II)(CH3CN)](ClO4)2 (1, tmpa = tris(2-pyridylmethyl)amine) and [(BzQ)Cu(II)(H2O)2](ClO4)2 (2, BzQ = bis(2-quinolinylmethyl)benzylamine)], act as efficient catalysts for the selective two-electron reduction of O2 by ferrocene derivatives in the presence of scandium triflate (Sc(OTf)3) in acetone, whereas 1 catalyzes the four-electron reduction of O2 by the same reductant in the presence of Brønsted acids such as triflic acid. Following formation of the peroxo-bridged dicopper(II) complex [(tmpa)Cu(II)(O2)Cu(II)(tmpa)](2+), the two-electron reduced product of O2 with Sc(3+) is observed to be scandium peroxide ([Sc(III)(O2(2-))](+)). In the presence of 3 equiv of hexamethylphosphoric triamide (HMPA), [Sc(III)(O2(2-))](+) was oxidized by [Fe(bpy)3](3+) (bpy = 2,2-bipyridine) to the known superoxide species [(HMPA)3Sc(III)(O2(•-))](2+) as detected by EPR spectroscopy. A kinetic study revealed that the rate-determining step of the catalytic cycle for the two-electron reduction of O2 with 1 is electron transfer from Fc* to 1 to give a cuprous complex which is highly reactive toward O2, whereas the rate-determining step with 2 is changed to the reaction of the cuprous complex with O2 following electron transfer from ferrocene derivatives to 2. The explanation for the change in catalytic O2-reaction stoichiometry from four-electron with Brønsted acids to two-electron reduction in the presence of Sc(3+) and also for the change in the rate-determining step is clarified based on a kinetics interrogation of the overall catalytic cycle as well as each step of the catalytic cycle with study of the observed effects of Sc(3+) on copper-oxygen intermediates.
Figures
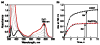






References
-
- Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L. Chem Rev. 2014;114:3659. - PMC - PubMed
- Kosman DJ. J Biol Inorg Chem. 2010;15:15. - PubMed
- Battaini G, Granata A, Monzani E, Gullotti M, Casella L. Adv Inorg Chem. 2006;58:185.
- Rosenzweig AC. Nat Chem. 2009;1:684. - PubMed
-
- McGuirl MA, Dooley DM. Copper Proteins with Type 2 Sites. In: King RB, editor. Encyclopedia of Inorganic Chemistry. 2. II. John Wiley & Sons Ltd; Chichester: 2005. pp. 1201–1225.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

