Positive and negative regulation of vertebrate separase by Cdk1-cyclin B1 may explain why securin is dispensable
- PMID: 25659430
- PMCID: PMC4367298
- DOI: 10.1074/jbc.M114.615310
Positive and negative regulation of vertebrate separase by Cdk1-cyclin B1 may explain why securin is dispensable
Abstract
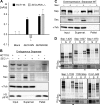
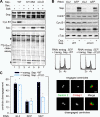
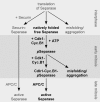
Sister chromatid cohesion is established during replication by entrapment of both dsDNAs within the cohesin ring complex. It is dissolved in anaphase when separase, a giant cysteine endopeptidase, cleaves the Scc1/Rad21 subunit of cohesin, thereby triggering chromosome segregation. Separase is held inactive by association with securin until this anaphase inhibitor is destroyed at the metaphase-to-anaphase transition by ubiquitin-dependent degradation. The relevant ubiquitin ligase, the anaphase-promoting complex/cyclosome, also targets cyclin B1, thereby causing inactivation of Cdk1 and mitotic exit. Although separase is essential, securin knock-out mice are surprisingly viable and fertile. Capitalizing on our previous finding that Cdk1-cyclin B1 can also bind and inhibit separase, we investigated whether this kinase might be suitable to maintain faithful timing and execution of anaphase in the absence of securin. We found that, similar to securin, Cdk1-cyclin B1 regulates separase in both a positive and negative manner. Although securin associates with nascent separase to co-translationally assist proper folding, Cdk1-cyclin B1 acts on native state separase. Upon entry into mitosis, Cdk1-cyclin B1-dependent phosphorylation of Ser-1126 renders separase prone to inactivation by aggregation/precipitation. Stable association of Cdk1-cyclin B1 with phosphorylated separase counteracts this tendency and stabilizes separase in an inhibited yet activatable state. These opposing effects are suited to prevent premature cleavage of cohesin in early mitosis while ensuring timely activation of separase by anaphase-promoting complex/cyclosome-dependent degradation of cyclin B1. Coupling sister chromatid separation with subsequent exit from mitosis by this simplified mode might have been the common scheme of mitotic control prior to the evolution of securin.
Keywords: Cdk1-Cyclin B1; Centriole Disengagement; Chromosomes; Cyclin-dependent Kinase (CDK); Cysteine Protease; Mitosis; Phosphorylation; Securin; Separase; Sister Chromatid Separation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Wirth K. G., Wutz G., Kudo N. R., Desdouets C., Zetterberg A., Taghybeeglu S., Seznec J., Ducos G. M., Ricci R., Firnberg N., Peters J. M., Nasmyth K. (2006) Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172, 847–860 - PMC - PubMed
-
- Uhlmann F., Wernic D., Poupart M. A., Koonin E. V., Nasmyth K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375–386 - PubMed
-
- Haering C. H., Farcas A. M., Arumugam P., Metson J., Nasmyth K. (2008) The cohesin ring concatenates sister DNA molecules. Nature 454, 297–301 - PubMed
-
- Zou H., McGarry T. J., Bernal T., Kirschner M. W. (1999) Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418–422 - PubMed
-
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. (1995) A 20 S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

