KATs in cancer: functions and therapies
- PMID: 25659580
- PMCID: PMC4530097
- DOI: 10.1038/onc.2014.453
KATs in cancer: functions and therapies
Abstract
Post-translational acetylation of lysines is most extensively studied in histones, but this modification is also found in many other proteins and is implicated in a wide range of biological processes in both the cell nucleus and the cytoplasm. Like phosphorylation, acetylation patterns and levels are often altered in cancer, therefore small molecule inhibition of enzymes that regulate acetylation and deacetylation offers much potential for inhibiting cancer cell growth, as does disruption of interactions between acetylated residues and 'reader' proteins. For more than a decade now, histone deacetylase inhibitors have been investigated for their ability to increase acetylation and promote expression of tumor suppressor genes. However, emerging evidence suggests that acetylation can also promote cancer, in part by enhancing the functions of oncogenic transcription factors. In this review, we focus on how acetylation of both histone and non-histone proteins may drive cancer, and we will discuss the implications of such changes on how patients are assigned to therapeutic agents. Finally, we will explore what the future holds in the design of small-molecule inhibitors for modulation of levels or functions of acetylation states.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
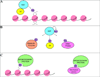

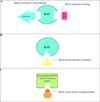
References
-
- Kuo MH, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

