Ligand-directed targeting of lymphatic vessels uncovers mechanistic insights in melanoma metastasis
- PMID: 25659743
- PMCID: PMC4345577
- DOI: 10.1073/pnas.1424994112
Ligand-directed targeting of lymphatic vessels uncovers mechanistic insights in melanoma metastasis
Abstract
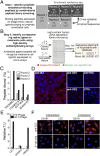
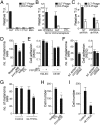
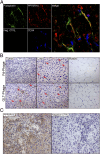
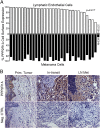
Metastasis is the most lethal step of cancer progression in patients with invasive melanoma. In most human cancers, including melanoma, tumor dissemination through the lymphatic vasculature provides a major route for tumor metastasis. Unfortunately, molecular mechanisms that facilitate interactions between melanoma cells and lymphatic vessels are unknown. Here, we developed an unbiased approach based on molecular mimicry to identify specific receptors that mediate lymphatic endothelial-melanoma cell interactions and metastasis. By screening combinatorial peptide libraries directly on afferent lymphatic vessels resected from melanoma patients during sentinel lymphatic mapping and lymph node biopsies, we identified a significant cohort of melanoma and lymphatic surface binding peptide sequences. The screening approach was designed so that lymphatic endothelium binding peptides mimic cell surface proteins on tumor cells. Therefore, relevant metastasis and lymphatic markers were biochemically identified, and a comprehensive molecular profile of the lymphatic endothelium during melanoma metastasis was generated. Our results identified expression of the phosphatase 2 regulatory subunit A, α-isoform (PPP2R1A) on the cell surfaces of both melanoma cells and lymphatic endothelial cells. Validation experiments showed that PPP2R1A is expressed on the cell surfaces of both melanoma and lymphatic endothelial cells in vitro as well as independent melanoma patient samples. More importantly, PPP2R1A-PPP2R1A homodimers occur at the cellular level to mediate cell-cell interactions at the lymphatic-tumor interface. Our results revealed that PPP2R1A is a new biomarker for melanoma metastasis and show, for the first time to our knowledge, an active interaction between the lymphatic vasculature and melanoma cells during tumor progression.
Keywords: cell surface peptide; cell–cell interaction; lymphatic targeting; phage display.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the B16 murine melanoma.Neoplasia. 2010 Feb;12(2):173-82. doi: 10.1593/neo.91604. Neoplasia. 2010. PMID: 20126475 Free PMC article.
-
Prognostic significance of tumor-associated lymphangiogenesis in malignant melanomas of the conjunctiva.Ophthalmology. 2011 Dec;118(12):2351-60. doi: 10.1016/j.ophtha.2011.05.025. Epub 2011 Aug 11. Ophthalmology. 2011. PMID: 21835473
-
Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma.Arch Dermatol Res. 2006 Feb;297(8):352-7. doi: 10.1007/s00403-005-0633-1. Epub 2006 Jan 3. Arch Dermatol Res. 2006. PMID: 16395613
-
Tumor lymphangiogenesis and melanoma metastasis.J Cell Physiol. 2008 Aug;216(2):347-54. doi: 10.1002/jcp.21494. J Cell Physiol. 2008. PMID: 18481261 Review.
-
Lymphatic biomarkers in primary melanomas as predictors of regional lymph node metastasis and patient outcomes.Pigment Cell Melanoma Res. 2013 May;26(3):326-37. doi: 10.1111/pcmr.12064. Epub 2013 Feb 4. Pigment Cell Melanoma Res. 2013. PMID: 23298266 Review.
Cited by
-
Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses.Int J Mol Sci. 2016 Dec 28;18(1):51. doi: 10.3390/ijms18010051. Int J Mol Sci. 2016. PMID: 28036019 Free PMC article. Review.
-
Melanoma-derived mediators can foster the premetastatic niche: crossroad to lymphatic metastasis.Trends Immunol. 2023 Sep;44(9):724-743. doi: 10.1016/j.it.2023.07.002. Epub 2023 Aug 10. Trends Immunol. 2023. PMID: 37573226 Free PMC article. Review.
-
Clinical utilities and biological characteristics of melanoma sentinel lymph nodes.World J Clin Oncol. 2016 Apr 10;7(2):174-88. doi: 10.5306/wjco.v7.i2.174. World J Clin Oncol. 2016. PMID: 27081640 Free PMC article. Review.
-
Identification of key genes involved in the pathogenesis of cutaneous melanoma using bioinformatics analysis.J Int Med Res. 2020 Jan;48(1):300060519895867. doi: 10.1177/0300060519895867. J Int Med Res. 2020. PMID: 31937175 Free PMC article.
-
Design and proof-of-concept for targeted phage-based COVID-19 vaccination strategies with a streamlined cold-free supply chain.bioRxiv [Preprint]. 2021 Mar 16:2021.03.15.435496. doi: 10.1101/2021.03.15.435496. bioRxiv. 2021. Update in: Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2105739118. doi: 10.1073/pnas.2105739118. PMID: 33758865 Free PMC article. Updated. Preprint.
References
-
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. - PubMed
-
- Stacker SA, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–191. - PubMed
-
- Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31(42):4499–4508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

