MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator
- PMID: 25659756
- PMCID: PMC4367483
- DOI: 10.1016/j.niox.2015.02.003
MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator
Abstract
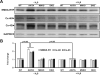
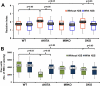
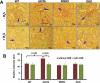
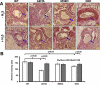
Previously we reported that matrix metalloproteinase-9 (MMP-9) plays an important role in extracellular matrix (ECM) remodeling in diabetic kidney. Induction of NMDA-R and dysregulation of connexins (Cxs) were also observed. We concluded that this was due to decreased H2S production by downregulation of CBS and CSE enzymes. However, the potential role of H2S to mitigate ECM dysregulation and renal dysfunction was not clearly understood. The present study was undertaken to determine whether H2S supplementation reduces MMP-9-induced ECM remodeling and dysfunction in diabetic kidney. Wild type (C57BL/6J), diabetic (Akita, C57BL/6J-Ins2(Akita)), MMP-9 knockout (MMP-9(-/-), M9KO) and double KO of Akita/MMP-9(-/-) (DKO) mice were treated without or with 0.005 g/l of NaHS (as a source of H2S) in drinking water for 30 days. Decreased tissue production and plasma content of H2S in Akita mice were ameliorated with H2S supplementation. Dysregulated expression of MMP-9, CBS, CSE, NMDA-R1 and Cxs-40, -43 was also normalized in Akita mice treated with H2S. In addition, increased renovascular resistive index (RI), ECM deposition, plasma creatinine, and diminished renal vascular density and cortical blood flow in Akita mice were normalized with H2S treatment. We conclude that diminished H2S production in renal tissue and plasma levels in diabetes mediates adverse renal remodeling, and H2S therapy improves renal function through MMP-9- and NMDA-R1-mediated pathway.
Keywords: Collagen–elastin; Connexins; Diabetes; Extracellular matrix; Hydrogen sulfide; Matrix metalloproteinase; Nephropathy; Renal microvasculature.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Tramonti G, Kanwar YS. Tubular biomarkers to assess progression of diabetic nephropathy. Kidney Int. 2011;79:1042–4. - PubMed
-
- Conway BR, Betz B, Sheldrake TA, Manning JR, Dunbar DR, Dobyns A, Hughes J, Mullins JJ. Tight blood glycaemic and blood pressure control in experimental diabetic nephropathy reduces extracellular matrix production without regression of fibrosis. Nephrology (Carlton) 2014;19:802–13. - PubMed
-
- Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, Kim SH, Han SH, Lee JE, Moon SJ, Ryu DR, Yoo TH, Han DS, Kang SW. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F200–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

