Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice
- PMID: 25659822
- PMCID: PMC4459023
- DOI: 10.1111/bph.13109
Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice
Abstract
Background and purpose: Endogenous glucocorticoids are pro-resolving mediators, an example of which is the endogenous glucocorticoid-regulated protein annexin A1 (ANXA1). Because silicosis is an occupational lung disease characterized by unabated inflammation and fibrosis, in this study we tested the therapeutic properties of the N-terminal ANXA1-derived peptide annexin 1-(2-26) (Ac2-26) on experimental silicosis.
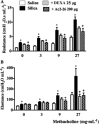
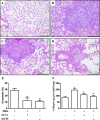
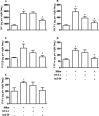
Experimental approach: Swiss-Webster mice were administered silica particles intranasally and were subsequently treated with intranasal peptide Ac2-26 (200 μg per mouse) or dexamethasone (25 μg per mouse) for 7 days, starting 6 h post-challenge. Ac2-26 abolished the leukocyte infiltration, collagen deposition, granuloma formation and generation of pro-inflammatory cytokines evoked by silica; these variables were only partially inhibited by dexamethasone.
Key results: A clear exacerbation of the silica-induced pathological changes was observed in ANXA1 knockout mice as compared with their wild-type (WT) littermate controls. Incubation of lung fibroblasts from WT mice with Ac2-26 in vitro reduced IL-13 or TGF-β-induced production of CCL2 (MCP-1) and collagen, but this peptide did not affect the production of CCL2 (MCP-1) by stimulated fibroblasts from formyl peptide receptor type 1 (FPR1) knockout mice. Ac2-26 also inhibited the production of CCL2 (MCP-1) from fibroblasts of FPR2 knockout mice.
Conclusions and implications: Collectively, our findings reveal novel protective properties of the ANXA1 derived peptide Ac2-26 on the inflammatory and fibrotic responses induced by silica, and suggest that ANXA1 mimetic agents might be a promising strategy as innovative anti-fibrotic approaches for the treatment of silicosis.
© 2015 The British Pharmacological Society.
Figures





References
-
- Arcangeli G, Cupelli V, Giuliano G. Effects of silica on human lung fibroblast in culture. Sci Total Environ. 2001;270:135–139. - PubMed
-
- Baroni T, Bodo M, D'Alessandro A, Conte C, Calvitti M, Muzi G, et al. Silica and its antagonistic effects on transforming growth factor-beta in lung fibroblast extracellular matrix production. J Investig Med. 2001;49:146–156. - PubMed
-
- Carneiro AP, Barreto SM, Siqueira AL, Cavariani F, Forastiere F. Continued exposure to silica after diagnosis of silicosis in Brazilian gold miners. Am J Ind Med. 2006;49:811–818. - PubMed
-
- Croxtall JD, Waheed S, Choudhury Q, Anand R, Flower RJ. N-terminal peptide fragments of lipocortin-1 inhibit A549 cell growth and block EGF-induced stimulation of proliferation. Int J Cancer. 1993;54:153–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

