Myotubularin-related proteins 3 and 4 interact with polo-like kinase 1 and centrosomal protein of 55 kDa to ensure proper abscission
- PMID: 25659891
- PMCID: PMC4390272
- DOI: 10.1074/mcp.M114.046086
Myotubularin-related proteins 3 and 4 interact with polo-like kinase 1 and centrosomal protein of 55 kDa to ensure proper abscission
Abstract
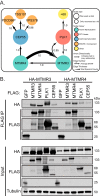
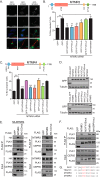
The myotubularins are a family of phosphatases that dephosphorylate the phosphatidylinositols phosphatidylinositol-3-phosphate and phosphatidylinositol-3,5-phosphate. Several family members are mutated in disease, yet the biological functions of the majority of myotubularins remain unknown. To gain insight into the roles of the individual enzymes, we have used affinity purification coupled to mass spectrometry to identify protein-protein interactions for the myotubularins. The myotubularin interactome comprises 66 high confidence (false discovery rate ≤1%) interactions, including 18 pairwise interactions between individual myotubularins. The results reveal a number of potential signaling contexts for this family of enzymes, including an intriguing, novel role for myotubularin-related protein 3 and myotubularin-related protein 4 in the regulation of abscission, the final step of mitosis in which the membrane bridge remaining between two daughter cells is cleaved. Both depletion and overexpression of either myotubularin-related protein 3 or myotubularin-related protein 4 result in abnormal midbody morphology and cytokinesis failure. Interestingly, myotubularin-related protein 3 and myotubularin-related protein 4 do not exert their effects through lipid regulation at the midbody, but regulate abscission during early mitosis, by interacting with the mitotic kinase polo-like kinase 1, and with centrosomal protein of 55 kDa (CEP55), an important regulator of abscission. Structure-function analysis reveals that, consistent with known intramyotubularin interactions, myotubularin-related protein 3 and myotubularin-related protein 4 interact through their respective coiled coil domains. The interaction between myotubularin-related protein 3 and polo-like kinase 1 relies on the divergent, nonlipid binding Fab1, YOTB, Vac1, and EEA1 domain of myotubularin-related protein 3, and myotubularin-related protein 4 interacts with CEP55 through a short GPPXXXY motif, analogous to endosomal sorting complex required for transport-I components. Disruption of any of these interactions results in abscission failure, by disrupting the proper recruitment of CEP55, and subsequently, of endosomal sorting complex required for transport-I, to the midbody. Our data suggest that myotubularin-related protein 3 and myotubularin-related protein 4 may act as a bridge between CEP55 and polo-like kinase 1, ensuring proper CEP55 phosphorylation and regulating CEP55 recruitment to the midbody. This work provides a novel role for myotubularin-related protein 3/4 heterodimers, and highlights the temporal and spatial complexity of the regulation of cytokinesis.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody.Oncotarget. 2016 May 24;7(21):30820-30. doi: 10.18632/oncotarget.9003. Oncotarget. 2016. PMID: 27127172 Free PMC article.
-
Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission.J Cell Biol. 2010 Nov 15;191(4):751-60. doi: 10.1083/jcb.201008108. J Cell Biol. 2010. PMID: 21079244 Free PMC article.
-
iASPP-PP1 complex is required for cytokinetic abscission by controlling CEP55 dephosphorylation.Cell Death Dis. 2018 May 1;9(5):528. doi: 10.1038/s41419-018-0561-6. Cell Death Dis. 2018. PMID: 29743530 Free PMC article.
-
Cytokinesis and cancer: Polo loves ROCK'n' Rho(A).J Genet Genomics. 2010 Mar;37(3):159-72. doi: 10.1016/S1673-8527(09)60034-5. J Genet Genomics. 2010. PMID: 20347825 Review.
-
Knowing when to cut and run: mechanisms that control cytokinetic abscission.Trends Cell Biol. 2013 Sep;23(9):433-41. doi: 10.1016/j.tcb.2013.04.006. Epub 2013 May 22. Trends Cell Biol. 2013. PMID: 23706391 Review.
Cited by
-
Myotubularin-Related Protein14 Prevents Neointima Formation and Vascular Smooth Muscle Cell Proliferation by Inhibiting Polo-Like Kinase1.J Am Heart Assoc. 2022 Nov;11(21):e026174. doi: 10.1161/JAHA.122.026174. Epub 2022 Oct 31. J Am Heart Assoc. 2022. PMID: 36314496 Free PMC article.
-
The Roles of Pseudophosphatases in Disease.Int J Mol Sci. 2021 Jun 28;22(13):6924. doi: 10.3390/ijms22136924. Int J Mol Sci. 2021. PMID: 34203203 Free PMC article. Review.
-
Beyond cytokinesis: the emerging roles of CEP55 in tumorigenesis.Oncogene. 2016 Feb 11;35(6):683-90. doi: 10.1038/onc.2015.128. Epub 2015 Apr 27. Oncogene. 2016. PMID: 25915844 Review.
-
A truncating mutation in CEP55 is the likely cause of MARCH, a novel syndrome affecting neuronal mitosis.J Med Genet. 2017 Jul;54(7):490-501. doi: 10.1136/jmedgenet-2016-104296. Epub 2017 Mar 6. J Med Genet. 2017. PMID: 28264986 Free PMC article.
-
Defining the Protein-Protein Interaction Network of the Human Protein Tyrosine Phosphatase Family.Mol Cell Proteomics. 2016 Sep;15(9):3030-44. doi: 10.1074/mcp.M116.060277. Epub 2016 Jul 18. Mol Cell Proteomics. 2016. PMID: 27432908 Free PMC article.
References
-
- Robinson F. L., Dixon J. E. (2006) Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 16, 403–412 - PubMed
-
- Hnia K., Vaccari I., Bolino A., Laporte J. (2012) Myotubularin phosphoinositide phosphatases: cellular functions and disease pathophysiology. Trends Mol. Med. 18, 317–327 - PubMed
-
- Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. (2004) Protein tyrosine phosphatases in the human genome. Cell 117, 699–711 - PubMed
-
- Lorenzo O., Urbé S., Clague M. J. (2006) Systematic analysis of myotubularins: heteromeric interactions, subcellular localization, and endosome related functions. J. Cell Sci. 119, 2953–2959 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

