Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension
- PMID: 25659899
- PMCID: PMC4385991
- DOI: 10.1152/ajplung.00155.2014
Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension
Abstract
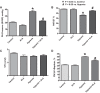
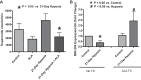
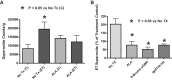
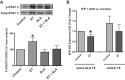
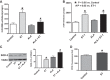
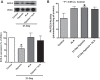
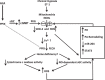
This study examines how heme biosynthesis modulation with δ-aminolevulinic acid (ALA) potentially functions to prevent 21-day hypoxia (10% oxygen)-induced pulmonary hypertension in mice and the effects of 24-h organoid culture with bovine pulmonary arteries (BPA) with the hypoxia and pulmonary hypertension mediator endothelin-1 (ET-1), with a focus on changes in superoxide and regulation of micro-RNA 204 (miR204) expression by src kinase phosphorylation of signal transducer and activator of transcription-3 (STAT3). The treatment of mice with ALA attenuated pulmonary hypertension (assessed through echo Doppler flow of the pulmonary valve, and direct measurements of right ventricular systolic pressure and right ventricular hypertrophy), increases in pulmonary arterial superoxide (detected by lucigenin), and decreases in lung miR204 and mitochondrial superoxide dismutase (SOD2) expression. ALA treatment of BPA attenuated ET-1-induced increases in mitochondrial superoxide (detected by MitoSox), STAT3 phosphorylation, and decreases in miR204 and SOD2 expression. Because ALA increases BPA protoporphyrin IX (a stimulator of guanylate cyclase) and cGMP-mediated protein kinase G (PKG) activity, the effects of the PKG activator 8-bromo-cGMP were examined and found to also attenuate the ET-1-induced increase in superoxide. ET-1 increased superoxide production and the detection of protoporphyrin IX fluorescence, suggesting oxidant conditions might impair heme biosynthesis by ferrochelatase. However, chronic hypoxia actually increased ferrochelatase activity in mouse pulmonary arteries. Thus, a reversal of factors increasing mitochondrial superoxide and oxidant effects that potentially influence remodeling signaling related to miR204 expression and perhaps iron availability needed for the biosynthesis of heme by the ferrochelatase reaction could be factors in the beneficial actions of ALA in pulmonary hypertension.
Keywords: endothelin; ferrochelatase; guanylate cyclase; micro-RNA 204; superoxide.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide.Am J Physiol Regul Integr Comp Physiol. 2014 Aug 15;307(4):R426-33. doi: 10.1152/ajpregu.00257.2013. Epub 2014 Jun 11. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24920729 Free PMC article.
-
Potential role of mitochondrial superoxide decreasing ferrochelatase and heme in coronary artery soluble guanylate cyclase depletion by angiotensin II.Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1439-47. doi: 10.1152/ajpheart.00859.2015. Epub 2016 Apr 1. Am J Physiol Heart Circ Physiol. 2016. PMID: 27037373 Free PMC article.
-
Endothelin-1 depletion of cartilage oligomeric matrix protein modulates pulmonary artery superoxide and iron metabolism-associated mitochondrial heme biosynthesis.Am J Physiol Lung Cell Mol Physiol. 2022 Oct 1;323(4):L400-L409. doi: 10.1152/ajplung.00534.2020. Epub 2022 Aug 9. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35943724 Free PMC article.
-
Redox regulation of responses to hypoxia and NO-cGMP signaling in pulmonary vascular pathophysiology.Ann N Y Acad Sci. 2010 Aug;1203:126-32. doi: 10.1111/j.1749-6632.2010.05557.x. Ann N Y Acad Sci. 2010. PMID: 20716294 Review.
-
In order for the light to shine so brightly, the darkness must be present-why do cancers fluoresce with 5-aminolaevulinic acid?Br J Cancer. 2019 Oct;121(8):631-639. doi: 10.1038/s41416-019-0516-4. Epub 2019 Aug 13. Br J Cancer. 2019. PMID: 31406300 Free PMC article. Review.
Cited by
-
Mitochondrial Iron in Human Health and Disease.Annu Rev Physiol. 2019 Feb 10;81:453-482. doi: 10.1146/annurev-physiol-020518-114742. Epub 2018 Nov 28. Annu Rev Physiol. 2019. PMID: 30485761 Free PMC article. Review.
-
Hypoxia-induced pulmonary hypertension in type 2 diabetic mice.Pulm Circ. 2017 Feb 1;7(1):175-185. doi: 10.1086/690206. eCollection 2017 Mar. Pulm Circ. 2017. PMID: 28680577 Free PMC article.
-
Iron Metabolism and Vascular Remodeling: Novel Insights Provided by Transferrin-1 Receptor Depletion in Mice With Pulmonary Hypertension.Am J Hypertens. 2016 Jun;29(6):676-8. doi: 10.1093/ajh/hpv175. Epub 2015 Nov 4. Am J Hypertens. 2016. PMID: 26541568 Free PMC article. No abstract available.
-
Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1α Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function.Stem Cells Dev. 2016 Jul 15;25(14):1084-94. doi: 10.1089/scd.2016.0072. Epub 2016 Jun 27. Stem Cells Dev. 2016. PMID: 27224420 Free PMC article.
-
Rotenone-stimulated superoxide release from mitochondrial complex I acutely augments L-type Ca2+ current in A7r5 aortic smooth muscle cells.Am J Physiol Heart Circ Physiol. 2016 May 1;310(9):H1118-28. doi: 10.1152/ajpheart.00889.2015. Epub 2016 Feb 12. Am J Physiol Heart Circ Physiol. 2016. PMID: 26873970 Free PMC article.
References
-
- Alhawaj R, Wolin MS. Aminolevulinic acid attenuates increases in pulmonary arterial superoxide elicited by organoid culture with endothelin-1 and exposure of mice to chronic hypoxia through mechanisms potentially involving protoporphyrin IX-elicited stimulation of soluble guanylate cyclase and cGMP protein kinase signaling (Abstract). FASEB J 27: lb701, 2013.
-
- Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121: 2661–2671, 2010. - PMC - PubMed
-
- Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts. Relevance to aging. J Biol Chem 276: 48410–48416, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

