Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure
- PMID: 25659904
- PMCID: PMC4398870
- DOI: 10.1152/ajplung.00276.2014
Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure
Abstract
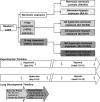
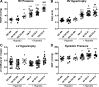
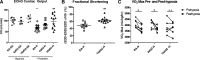
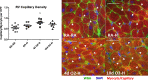
The development of pulmonary hypertension (PH) requires multiple pulmonary vascular insults, yet the role of early oxygen therapy as an initial pulmonary vascular insult remains poorly defined. Here, we employ a two-hit model of PH, utilizing postnatal hyperoxia followed by adult hypoxia exposure, to evaluate the role of early hyperoxic lung injury in the development of later PH. Sprague-Dawley pups were exposed to 90% oxygen during postnatal days 0-4 or 0-10 or to room air. All pups were then allowed to mature in room air. At 10 wk of age, a subset of rats from each group was exposed to 2 wk of hypoxia (Patm = 362 mmHg). Physiological, structural, and biochemical endpoints were assessed at 12 wk. Prolonged (10 days) postnatal hyperoxia was independently associated with elevated right ventricular (RV) systolic pressure, which worsened after hypoxia exposure later in life. These findings were only partially explained by decreases in lung microvascular density. Surprisingly, postnatal hyperoxia resulted in robust RV hypertrophy and more preserved RV function and exercise capacity following adult hypoxia compared with nonhyperoxic rats. Biochemically, RVs from animals exposed to postnatal hyperoxia and adult hypoxia demonstrated increased capillarization and a switch to a fetal gene pattern, suggesting an RV more adept to handle adult hypoxia following postnatal hyperoxia exposure. We concluded that, despite negative impacts on pulmonary artery pressures, postnatal hyperoxia exposure may render a more adaptive RV phenotype to tolerate late pulmonary vascular insults.
Keywords: atrial natriuretic peptide; capillarization; prematurity; pulmonary hypertension; right ventricular adaptation.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Postnatal Hyperoxia Exposure Durably Impairs Right Ventricular Function and Mitochondrial Biogenesis.Am J Respir Cell Mol Biol. 2017 May;56(5):609-619. doi: 10.1165/rcmb.2016-0256OC. Am J Respir Cell Mol Biol. 2017. PMID: 28129517 Free PMC article.
-
Right ventricular cyclic nucleotide signaling is decreased in hyperoxia-induced pulmonary hypertension in neonatal mice.Am J Physiol Heart Circ Physiol. 2015 Jun 15;308(12):H1575-82. doi: 10.1152/ajpheart.00569.2014. Epub 2015 Apr 10. Am J Physiol Heart Circ Physiol. 2015. PMID: 25862831 Free PMC article.
-
Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia.Pediatr Res. 2016 Dec;80(6):894-902. doi: 10.1038/pr.2016.164. Epub 2016 Aug 10. Pediatr Res. 2016. PMID: 27509009
-
Can maternal DHA supplementation offer long-term protection against neonatal hyperoxic lung injury?Am J Physiol Lung Cell Mol Physiol. 2015 Dec 15;309(12):L1383-6. doi: 10.1152/ajplung.00313.2015. Epub 2015 Sep 11. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26361877 Free PMC article. Review.
-
Oxygen, the lung and the diver: friends and foes?Eur Respir Rev. 2016 Dec;25(142):496-505. doi: 10.1183/16000617.0049-2016. Eur Respir Rev. 2016. PMID: 27903670 Free PMC article. Review.
Cited by
-
Exaggerated Cardiac Contractile Response to Hypoxia in Adults Born Preterm.J Clin Med. 2021 Mar 10;10(6):1166. doi: 10.3390/jcm10061166. J Clin Med. 2021. PMID: 33802149 Free PMC article.
-
Exercise and hypoxia unmask pulmonary vascular disease and right ventricular dysfunction in a 10- to 12-week-old swine model of neonatal oxidative injury.J Physiol. 2022 Sep;600(17):3931-3950. doi: 10.1113/JP282906. Epub 2022 Aug 11. J Physiol. 2022. PMID: 35862359 Free PMC article.
-
Respiratory responses to hypoxia during rest and exercise in individuals born pre-term: a state-of-the-art review.Eur J Appl Physiol. 2022 Sep;122(9):1991-2003. doi: 10.1007/s00421-022-04965-9. Epub 2022 May 19. Eur J Appl Physiol. 2022. PMID: 35589858 Review.
-
Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes.J Clin Med. 2017 Jan 6;6(1):4. doi: 10.3390/jcm6010004. J Clin Med. 2017. PMID: 28067830 Free PMC article. Review.
-
Maladaptive functional changes in alveolar fibroblasts due to perinatal hyperoxia impair epithelial differentiation.JCI Insight. 2022 Mar 8;7(5):e152404. doi: 10.1172/jci.insight.152404. JCI Insight. 2022. PMID: 35113810 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

