Elucidating the mechanism by which compensatory mutations rescue an HIV-1 matrix mutant defective for gag membrane targeting and envelope glycoprotein incorporation
- PMID: 25659909
- PMCID: PMC4844178
- DOI: 10.1016/j.jmb.2015.01.018
Elucidating the mechanism by which compensatory mutations rescue an HIV-1 matrix mutant defective for gag membrane targeting and envelope glycoprotein incorporation
Abstract
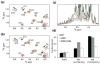
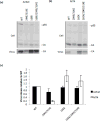
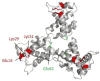
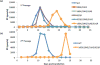
The matrix (MA) domain of the human immunodeficiency virus (HIV) 1 Gag is responsible for Gag targeting to the plasma membrane where virions assemble. MA also plays a role in the incorporation of the viral envelope (Env) glycoproteins and can influence particle infectivity post-maturation and post-entry. A highly basic region of MA targets Gag to the plasma membrane via specific binding to phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]. This binding also triggers exposure of an amino-terminal myristate moiety, which anchors Gag to the membrane. An MA mutant deficient for PI(4,5)P2 binding, 29KE/31KE, has been shown to mislocalize within the cell, leading to particle assembly in a multivesicular body compartment and defective release of cell-free particles in HeLa and 293T cells. Despite the defect in virus production in these cells, release of the 29KE/31KE mutant is not significantly reduced in primary T cells, macrophages and Jurkat T cells. 29KE/31KE virions also display an infectivity defect associated with impaired Env incorporation, irrespective of the producer cell line. Here we examine the properties of 29KE/31KE by analyzing compensatory mutations obtained by a viral adaptation strategy. The MA mutant 16EK restores virus release through enhanced membrane binding. 16EK also influences the infectivity defect, in combination with an additional MA mutant, 62QR. Additionally, the 29KE/31KE MA mutant displays a defect in proteolytic cleavage of the murine leukemia virus Env cytoplasmic tail in pseudotyped virions. Our findings elucidate the mechanism whereby an MA mutant defective in PI(4,5)P2 binding can be rescued and highlight the ability of MA to influence Env glycoprotein function.
Keywords: A-MLV; Gag; cytoplasmic tail; pseudotyping; retrovirus.
Published by Elsevier Ltd.
Figures



References
-
- Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

