Chemotherapeutic potential of diazeniumdiolate-based aspirin prodrugs in breast cancer
- PMID: 25659932
- PMCID: PMC4441830
- DOI: 10.1016/j.freeradbiomed.2015.01.029
Chemotherapeutic potential of diazeniumdiolate-based aspirin prodrugs in breast cancer
Abstract
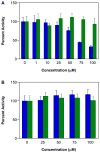
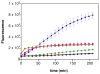
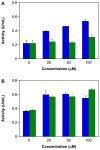
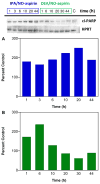
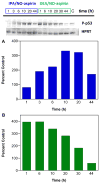
Diazeniumdiolate-based aspirin prodrugs have previously been shown to retain the anti-inflammatory properties of aspirin while protecting against the common side effect of stomach ulceration. Initial analysis of two new prodrugs of aspirin that also release either nitroxyl (HNO) or nitric oxide (NO) demonstrated increased cytotoxicity toward human lung carcinoma cells compared to either aspirin or the parent nitrogen oxide donor. In addition, cytotoxicity was significantly lower in endothelial cells, suggesting cancer-specific sensitivity. To assess the chemotherapeutic potential of these new prodrugs in treatment of breast cancer, we studied their effect both in cultured cells and in a nude mouse model. Both prodrugs reduced growth of breast adenocarcinoma cells more effectively than the parent compounds while not being appreciably cytotoxic in a related nontumorigenic cell line (MCF-10A). The HNO donor also was more cytotoxic than the related NO donor. The basis for the observed specificity was investigated in terms of impact on metabolism, DNA damage and repair, apoptosis, angiogenesis and metastasis. The results suggest a significant pharmacological potential for treatment of breast cancer.
Keywords: Anti-inflammatory; Anticancer; Aspirin; DEA/NO; Diazeniumdiolate; IPA/NO; NONOate; NSAID; Nitric oxide; Nitroxyl.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
A patent on use of IPA/NO-aspirin is held by KMM, DAW and DB.
Figures









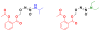
References
-
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. - PubMed
-
- Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. - PubMed
-
- The SALT Collaborative Group. Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338:1345–1349. - PubMed
-
- Cryer B, Luk G, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterol. 1999;117:17–25. - PubMed
-
- Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992;327:749–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

