The effects of sarcolipin over-expression in mouse skeletal muscle on metabolic activity
- PMID: 25660043
- PMCID: PMC4362768
- DOI: 10.1016/j.abb.2015.01.027
The effects of sarcolipin over-expression in mouse skeletal muscle on metabolic activity
Abstract
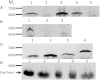
Studies in sarcolipin knockout mice have led to the suggestion that skeletal muscle sarcolipin plays a role in thermogenesis. The mechanism proposed is uncoupling of the sarcoplasmic reticulum calcium pump. However, in other work sarcolipin was not detected in mouse skeletal tissue. We have therefore measured sarcolipin levels in mouse skeletal muscle using semi-quantitative western blotting and synthetic mouse sarcolipin. Sarcolipin levels were so low that it is unlikely that knocking out sarcolipin would have a measurable effect on thermogenesis by SERCA. In addition, overexpression of neither wild type nor FLAG-tagged variants of mouse sarcolipin in transgenic mice had any major significant effects on body mass, energy expenditure, even when mice were fed on a high fat diet.
Keywords: Energy expenditure; High fat diet; Obesity; SERCA; Sarcolipin; Thermogenesis.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

