Acetyl-L-carnitine increases mitochondrial protein acetylation in the aged rat heart
- PMID: 25660059
- PMCID: PMC4712450
- DOI: 10.1016/j.mad.2015.01.003
Acetyl-L-carnitine increases mitochondrial protein acetylation in the aged rat heart
Abstract
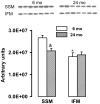
Previously we showed that in vivo treatment of elderly Fisher 344 rats with acetylcarnitine abolished the age-associated defect in respiratory chain complex III in interfibrillar mitochondria and improved the functional recovery of the ischemic/reperfused heart. Herein, we explored mitochondrial protein acetylation as a possible mechanism for acetylcarnitine's effect. In vivo treatment of elderly rats with acetylcarnitine restored cardiac acetylcarnitine content and increased mitochondrial protein lysine acetylation and increased the number of lysine-acetylated proteins in cardiac subsarcolemmal and interfibrillar mitochondria. Enzymes of the tricarboxylic acid cycle, mitochondrial β-oxidation, and ATP synthase of the respiratory chain showed the greatest acetylation. Acetylation of isocitrate dehydrogenase, long-chain acyl-CoA dehydrogenase, complex V, and aspartate aminotransferase was accompanied by decreased catalytic activity. Several proteins were found to be acetylated only after treatment with acetylcarnitine, suggesting that exogenous acetylcarnitine served as the acetyl-donor. Two-dimensional fluorescence difference gel electrophoresis analysis revealed that acetylcarnitine treatment also induced changes in mitochondrial protein amount; a two-fold or greater increase/decrease in abundance was observed for thirty one proteins. Collectively, our data provide evidence for the first time that in the aged rat heart in vivo administration of acetylcarnitine provides acetyl groups for protein acetylation and affects the amount of mitochondrial proteins.
Keywords: Acetylcarnitine; Aging; Heart; Mitochondria; Protein acetylation.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures
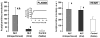



References
-
- Abbas AS, Wu G, Schulz H. Carnitine acetyltransferase is not a cytosolic enzyme in rat heart and therefore cannot function in the energy-linked regulation of cardiac fatty acid oxidation. J Mol Cell Cardiol. 1998;30:1305–1309. 1998. - PubMed
-
- Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signaling. Cardiovasc Res. 2014;103:485–497. - PMC - PubMed
-
- Ames BN. Optimal micronutrients delay mitochondrial decay and age-associated diseases. Mech Ageing Dev. 2010;131:473–479. - PubMed
-
- Bergmeyer HU, Bernt E. Glutamate-oxaloacetate transaminase in Methods of enzymatic analysis. 2. Vol. 2. Verlag Chemie/Academic Press; 1974. pp. 727–733.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

