Pharmacological modulation of autophagy to protect cardiomyocytes according to the time windows of ischaemia/reperfusion
- PMID: 25660104
- PMCID: PMC4459024
- DOI: 10.1111/bph.13111
Pharmacological modulation of autophagy to protect cardiomyocytes according to the time windows of ischaemia/reperfusion
Abstract
Background and purpose: Targeted modulation of autophagy induced by myocardial ischaemia/reperfusion has been the subject of intensive investigation, but it is debatable whether autophagy is beneficial or harmful. Hence, we evaluated the effects of pharmacological manipulation of autophagy on the survival of cardiomyocytes in different time windows of ischaemia/reperfusion.
Experimental approach: We examined the autophagy and apoptosis in cardiomyocytes subjected to different durations of anoxia/re-oxygenation or ischaemia/reperfusion, and evaluated the effects of the autophagic enhancer rapamycin and inhibitor wortmannin on cell survival.
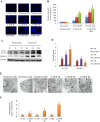
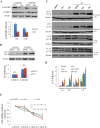
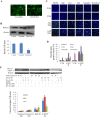
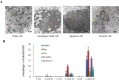
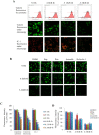
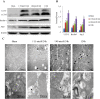
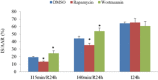
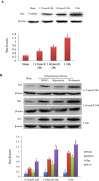
Key results: In neonatal rat cardiomyocytes (NRCs) or murine hearts, autophagy was increased in response to anoxia/reoxygenation or ischaemia/reperfusion in a time-dependent manner. Rapamycin-enhanced autophagy in NRCs led to higher cell viability and less apoptosis when anoxia was sustained for ≦ 6 h. When anoxia was prolonged to 12 h, rapamycin did not increase cell viability, induced less apoptosis and more autophagic cell death. When anoxia was prolonged to 24 h, rapamycin increased autophagic cell death, while wortmannin reduced autophagic cell death and apoptosis. Similar results were obtained in mice subjected to ischaemia/reperfusion. Rapamycin inhibited the opening of mitochondrial transition pore in NRCs exposed to 6 h anoxia/4 h re-oxygenation but did not exert any effect when anoxia was extended to 24 h. Similarly, rapamycin reduced the myocardial expression of Bax in mice subjected to short-time ischaemia, but this effect disappeared when ischaemia was extended to 24 h.
Conclusions and implications: The cardioprotection of autophagy is context-dependent and therapies involving the modification of autophagy should be determined according to the duration of ischaemia/reperfusion.
© 2015 The British Pharmacological Society.
Figures
References
-
- Aki T, Yamaguchi K, Fujimiya T, Mizukami Y. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene. 2003;22:8529–8535. - PubMed
-
- Cao X, Chen A, Yang P, Song X, Liu Y, Li Z, et al. Alpha-lipoic acid protects cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. Biochem Biophys Res Commun. 2013;441:935–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

