In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure
- PMID: 25660405
- PMCID: PMC4375942
- DOI: 10.1016/j.stemcr.2015.01.001
In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure
Abstract
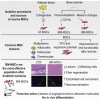
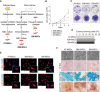
Over the last several years, mesenchymal stromal cells (MSCs) have been isolated from different tissues following a variety of different procedures. Here, we comparatively assess the ex vivo and in vivo properties of MSCs isolated from either adipose tissue or bone marrow by different purification protocols. After MSC transplantation into a mouse model of hindlimb ischemia, clinical and histological analysis revealed that bone marrow MSCs purified on adhesive substrates exerted the best therapeutic activity, preserving tissue viability and promoting formation of new arterioles without directly transdifferentiating into vascular cells. In keeping with these observations, these cells abundantly expressed cytokines involved in vessel maturation and cell retention. These findings indicate that the choice of MSC source and purification protocol is critical in determining the therapeutic potential of these cells and warrant the standardization of an optimal MSC isolation procedure in order to select the best conditions to move forward to more effective clinical experimentation.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Abbott J.D., Huang Y., Liu D., Hickey R., Krause D.S., Giordano F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. - PubMed
-
- Cho H.H., Kim Y.J., Kim J.T., Song J.S., Shin K.K., Bae Y.C., Jung J.S. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell. Physiol. Biochem. 2009;24:511–518. - PubMed
-
- Gerthoffer W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007;100:607–621. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

