Optogenetic induction of contractile ability in immature C2C12 myotubes
- PMID: 25661648
- PMCID: PMC4650824
- DOI: 10.1038/srep08317
Optogenetic induction of contractile ability in immature C2C12 myotubes
Abstract
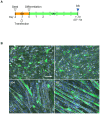
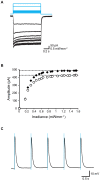
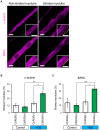
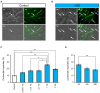
Myoblasts can be differentiated into multinucleated myotubes, which provide a well-established and reproducible muscle cell model for skeletal myogenesis in vitro. However, under conventional differentiation conditions, each myotube rarely exhibits robust contraction as well as sarcomere arrangement. Here, we applied trains of optical stimulation (OS) to C2C12 myotubes, which were genetically engineered to express a channelrhodopsin variant, channelrhodopsin-green receiver (ChRGR), to investigate whether membrane depolarization facilitates the maturation of myotubes. We found that light pulses induced membrane depolarization and evoked action potentials in ChRGR-expressing myotubes. Regular alignments of sarcomeric proteins were patterned periodically after OS training. In contrast, untrained control myotubes rarely exhibited the striated patterns. OS-trained and untrained myotubes also differed in terms of their resting potential. OS training significantly increased the number of contractile myotubes. Treatment with nifedipine during OS training significantly decreased the fraction of contractile myotubes, whereas tetrodotoxin was less effective. These results suggest that oscillations of membrane potential and intracellular Ca(2+) accompanied by OS promoted sarcomere assembly and the development of contractility during the myogenic process. These results also suggest that optogenetic techniques could be used to manipulate the activity-dependent process during myogenic development.
Figures


References
-
- Booth F. W., Chakravarthy M. V., Gordon S. E. & Spangenburg E. E. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 93, 3–30 (2002). - PubMed
-
- Flück M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J. Exp. Biol. 209, 2239–2248 (2006). - PubMed
-
- Sketelj J. et al. Specific impulse patterns regulate acetylcholinesterase activity in skeletal muscles of rats and rabbits. J. Neurosci. Res. 47, 49–57 (1997). - PubMed
-
- Freud-Silverberg M. & Shainberg A. Electric stimulation regulates the level of Ca-channels in chick muscle culture. Neurosci. Lett. 151, 104–106 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

