Structure of the vacuolar H+-ATPase rotary motor reveals new mechanistic insights
- PMID: 25661654
- PMCID: PMC4353692
- DOI: 10.1016/j.str.2014.12.016
Structure of the vacuolar H+-ATPase rotary motor reveals new mechanistic insights
Abstract
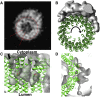
Vacuolar H(+)-ATPases are multisubunit complexes that operate with rotary mechanics and are essential for membrane proton transport throughout eukaryotes. Here we report a ∼ 1 nm resolution reconstruction of a V-ATPase in a different conformational state from that previously reported for a lower-resolution yeast model. The stator network of the V-ATPase (and by implication that of other rotary ATPases) does not change conformation in different catalytic states, and hence must be relatively rigid. We also demonstrate that a conserved bearing in the catalytic domain is electrostatic, contributing to the extraordinarily high efficiency of rotary ATPases. Analysis of the rotor axle/membrane pump interface suggests how rotary ATPases accommodate different c ring stoichiometries while maintaining high efficiency. The model provides evidence for a half channel in the proton pump, supporting theoretical models of ion translocation. Our refined model therefore provides new insights into the structure and mechanics of the V-ATPases.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures





References
-
- Abrahams J.P., Leslie A.G., Lutter R., Walker J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
-
- Angevine C.M., Herold K.A.G., Vincent O.D., Fillingame R.H. Aqueous access pathways in ATP synthase subunit A—reactivity of cysteine substituted into transmembrane helices 1, 3, and 5. J. Biol. Chem. 2007;282:9001–9007. - PubMed
-
- Arai S., Saijo S., Suzuki K., Mizutani K., Kakinuma Y., Ishizuka-Katsura Y., Ohsawa N., Terada T., Shirouzu M., Yokoyama S. Rotation mechanism of Enterococcus hirae V1-ATPase based on asymmetric crystal structures. Nature. 2013;493:703–707. - PubMed
-
- Beltran C., Nelson N. The membrane sector of vacuolar H(+)-ATPase by itself is impermeable to protons. Acta Physiol. Scand. Suppl. 1992;607:41–47. - PubMed
-
- Benlekbir S., Bueler S.A., Rubinstein J.L. Structure of the vacuolar-type ATPase from Saccharomyces cerevisiae at 11Å resolution. Nat. Struct. Mol. Biol. 2012;19:1356–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

