RNA circularization strategies in vivo and in vitro
- PMID: 25662225
- PMCID: PMC4344496
- DOI: 10.1093/nar/gkv045
RNA circularization strategies in vivo and in vitro
Abstract
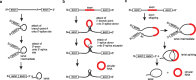
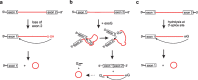
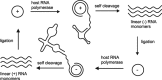
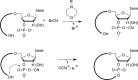
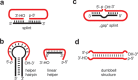
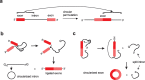
In the plenitude of naturally occurring RNAs, circular RNAs (circRNAs) and their biological role were underestimated for years. However, circRNAs are ubiquitous in all domains of life, including eukaryotes, archaea, bacteria and viruses, where they can fulfill diverse biological functions. Some of those functions, as for example playing a role in the life cycle of viral and viroid genomes or in the maturation of tRNA genes, have been elucidated; other putative functions still remain elusive. Due to the resistance to exonucleases, circRNAs are promising tools for in vivo application as aptamers, trans-cleaving ribozymes or siRNAs. How are circRNAs generated in vivo and what approaches do exist to produce ring-shaped RNAs in vitro? In this review we illustrate the occurrence and mechanisms of RNA circularization in vivo, survey methods for the generation of circRNA in vitro and provide appropriate protocols.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
-
- Flores R., Grubb D., Elleuch A., Nohales M.A., Delgado S., Gago S. Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol. 2011;8:200–206. - PubMed
-
- Kos A., Dijkema R., Arnberg A.C., van der Meide P.H., Schellekens H. The hepatitis delta virus possesses a circular RNA. Nature. 1986;323:558–560. - PubMed
-
- Lykke-Andersen J., Aagaard C., Semionenkov M., Garrett R.A. Archaeal introns: splicing, intercellular mobility and evolution. Trends Biochem. Sci. 1997;22:326–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

