Crucial role for the VWF A1 domain in binding to type IV collagen
- PMID: 25662333
- PMCID: PMC4383803
- DOI: 10.1182/blood-2014-11-610824
Crucial role for the VWF A1 domain in binding to type IV collagen
Abstract
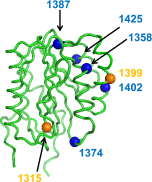
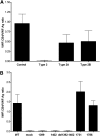
Von Willebrand factor (VWF) contains binding sites for platelets and for vascular collagens to facilitate clot formation at sites of injury. Although previous work has shown that VWF can bind type IV collagen (collagen 4), little characterization of this interaction has been performed. We examined the binding of VWF to collagen 4 in vitro and extended this characterization to a murine model of defective VWF-collagen 4 interactions. The interactions of VWF and collagen 4 were further studied using plasma samples from a large study of both healthy controls and subjects with different types of von Willebrand disease (VWD). Our results show that collagen 4 appears to bind VWF exclusively via the VWF A1 domain, and that specific sequence variations identified through VWF patient samples and through site-directed mutagenesis in the VWF A1 domain can decrease or abrogate this interaction. In addition, VWF-dependent platelet binding to collagen 4 under flow conditions requires an intact VWF A1 domain. We observed that decreased binding to collagen 4 was associated with select VWF A1 domain sequence variations in type 1 and type 2M VWD. This suggests an additional mechanism through which VWF variants may alter hemostasis.
© 2015 by The American Society of Hematology.
Figures


References
-
- Mohri H, Fujimura Y, Shima M, et al. Structure of the von Willebrand factor domain interacting with glycoprotein Ib. J Biol Chem. 1988;263(34):17901–17904. - PubMed
-
- Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262(28):13835–13841. - PubMed
-
- Ribba AS, Loisel I, Lavergne JM, et al. Ser968Thr mutation within the A3 domain of von Willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86(3):848–854. - PubMed
-
- Riddell AF, Gomez K, Millar CM, et al. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von Willebrand factor. Blood. 2009;114(16):3489–3496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

