Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis
- PMID: 25662776
- PMCID: PMC6742501
- DOI: 10.1016/S1474-4422(14)70324-2
Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis
Erratum in
- Lancet Neurol. 2015 Dec;14(12):1151. Binetti, Giuliano [added]
Abstract
Background: Frontotemporal dementia is a highly heritable neurodegenerative disorder. In about a third of patients, the disease is caused by autosomal dominant genetic mutations usually in one of three genes: progranulin (GRN), microtubule-associated protein tau (MAPT), or chromosome 9 open reading frame 72 (C9orf72). Findings from studies of other genetic dementias have shown neuroimaging and cognitive changes before symptoms onset, and we aimed to identify whether such changes could be shown in frontotemporal dementia.
Methods: We recruited participants to this multicentre study who either were known carriers of a pathogenic mutation in GRN, MAPT, or C9orf72, or were at risk of carrying a mutation because a first-degree relative was a known symptomatic carrier. We calculated time to expected onset as the difference between age at assessment and mean age at onset within the family. Participants underwent a standardised clinical assessment and neuropsychological battery. We did MRI and generated cortical and subcortical volumes using a parcellation of the volumetric T1-weighted scan. We used linear mixed-effects models to examine whether the association of neuropsychology and imaging measures with time to expected onset of symptoms differed between mutation carriers and non-carriers.
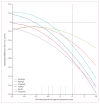
Findings: Between Jan 30, 2012, and Sept 15, 2013, we recruited participants from 11 research sites in the UK, Italy, the Netherlands, Sweden, and Canada. We analysed data from 220 participants: 118 mutation carriers (40 symptomatic and 78 asymptomatic) and 102 non-carriers. For neuropsychology measures, we noted the earliest significant differences between mutation carriers and non-carriers 5 years before expected onset, when differences were significant for all measures except for tests of immediate recall and verbal fluency. We noted the largest Z score differences between carriers and non-carriers 5 years before expected onset in tests of naming (Boston Naming Test -0·7; SE 0·3) and executive function (Trail Making Test Part B, Digit Span backwards, and Digit Symbol Task, all -0·5, SE 0·2). For imaging measures, we noted differences earliest for the insula (at 10 years before expected symptom onset, mean volume as a percentage of total intracranial volume was 0·80% in mutation carriers and 0·84% in non-carriers; difference -0·04, SE 0·02) followed by the temporal lobe (at 10 years before expected symptom onset, mean volume as a percentage of total intracranial volume 8·1% in mutation carriers and 8·3% in non-carriers; difference -0·2, SE 0·1).
Interpretation: Structural imaging and cognitive changes can be identified 5-10 years before expected onset of symptoms in asymptomatic adults at risk of genetic frontotemporal dementia. These findings could help to define biomarkers that can stage presymptomatic disease and track disease progression, which will be important for future therapeutic trials.
Funding: Centres of Excellence in Neurodegeneration.
Copyright © 2015 Rohrer et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
JDR is funded by a National Institute for Health Research Rare Disease Translational Research Collaboration Fellowship. SAR is supported by a Vici grant from the Netherlands Organization for Scientific Research. MM is supported by the Department of Medicine, Sunnybrook Health Sciences Centre, and the Sunnybrook Foundation. He is funded by the Canadian Institutes of Health Research and the Ontario Research Fund. He reports consultancy fees from Novartis, Teva, Union Chimique Belge Canada, General Electric Healthcare, and Bioscape Medical Imaging CRO all outside the submitted work. He also holds a US patent of a pharmacogenetic test for Parkinson’s disease. MF receives support from the Saul A Silverman Family Foundation as a Canada International Scientific Exchange Program and Morris Kerzner Memorial Fund, and is listed on a provisional patent related to methods and kits for differential diagnosis of Alzheimer’s disease versus frontotemporal dementia with blood biomarkers. ER is funded by the Weston Brain Institute and Ontario Research Fund. RL Jr is funded by Réseau de médecine génétique appliquée, Fonds de recherche du Québec—Santé (FRQS). FT, RG, and LB are funded by the Italian Ministry of Health. DG, ES, AA, and GF are supported by the Fondazione Monzino and Italian Ministry of Health, Ricerca Corrente. JBR is supported by a Wellcome Trust Senior Research Fellowship (088324). JBR and IC-G are supported by the National Institute for Health Cambridge Biomedical Research Centre and Biomedical Research Unit in Dementia. BN is funded by Cassa di Risparmio di Pistoia e Pescia (CRPT 2013/0347). SS is funded by Cassa di Risparmio di Firenze (CRF 2013/0199) and the Ministry of Health RF-2010-2319722. JDW is funded by a Wellcome Trust Senior Clinical Fellowship (091673/Z/10/Z). NCF and MNR are National Institute for Health Research Senior Investigators. NCF also reports consultancy fees (all paid to University College London) from Janssen/Pfizer, General Electric Healthcare, IXICO, Johnson & Johnson, Genzyme (Sonofi company), Eisai, Janssen Alzheimer’s Immunotherapy Research and Development, Lilly Research Laboratories (AVID), Eli Lilly, and Novartis Pharma AG all outside the submitted work. He also has a patent QA Box issued. The Dementia Research Centre at University College London is an Alzheimer’s Research UK coordinating centre and has received equipment funded by Alzheimer’s Research UK and Brain Research Trust. MNR also reports fees (paid to University College London) for serving on a Data Monitoring Committee for Servier outside the submitted work. SEB reports personal fees from Pfizer, GlaxoSmithKline, Novartis, Roche, Eli Lilly, Pfizer, Eisai, Boehringer Ingelheim, General Electric Healthcare, and Novartis, and grants from Pfizer, Elan/Transition Therapeutics Ireland Ltd, Roche, Eli Lilly, General Electric Healthcare, and Lundbeck all outside the submitted work. SO is funded by the Engineering and Physical Sciences Research Council (EP/H046410/1, EP/J020990/1, EP/K005278), the Medical Research Council (MR/J01107X/1), the EU-FP7 project VPH-DARE@IT (FP7-ICT-2011-9-601055), and the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269). All other authors declare no competing interests.
Figures
Comment in
-
Frontotemporal dementia: a peek under its invisibility cloak.Lancet Neurol. 2015 Mar;14(3):236-7. doi: 10.1016/S1474-4422(15)70019-0. Epub 2015 Feb 4. Lancet Neurol. 2015. PMID: 25662775 No abstract available.
References
-
- Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82:476–86. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_U123160651/MRC_/Medical Research Council/United Kingdom
- CAPMC/ CIHR/Canada
- MC_U105597119/MRC_/Medical Research Council/United Kingdom
- MR/J01107X/1/MRC_/Medical Research Council/United Kingdom
- 091673/WT_/Wellcome Trust/United Kingdom
- MR/J009482/1/MRC_/Medical Research Council/United Kingdom
- MR/M501724/1/MRC_/Medical Research Council/United Kingdom
- MR/M008525/1/MRC_/Medical Research Council/United Kingdom
- MR/M009041/1/MRC_/Medical Research Council/United Kingdom
- MR/M024873/1/MRC_/Medical Research Council/United Kingdom
- 088324/WT_/Wellcome Trust/United Kingdom
- MR/M023664/1/MRC_/Medical Research Council/United Kingdom
- MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- 103838/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

