Targeting Enterococcus faecalis biofilms with phage therapy
- PMID: 25662974
- PMCID: PMC4375334
- DOI: 10.1128/AEM.00096-15
Targeting Enterococcus faecalis biofilms with phage therapy
Abstract
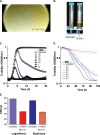
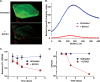
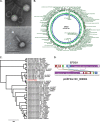
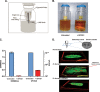
Phage therapy has been proven to be more effective, in some cases, than conventional antibiotics, especially regarding multidrug-resistant biofilm infections. The objective here was to isolate an anti-Enterococcus faecalis bacteriophage and to evaluate its efficacy against planktonic and biofilm cultures. E. faecalis is an important pathogen found in many infections, including endocarditis and persistent infections associated with root canal treatment failure. The difficulty in E. faecalis treatment has been attributed to the lack of anti-infective strategies to eradicate its biofilm and to the frequent emergence of multidrug-resistant strains. To this end, an anti-E. faecalis and E. faecium phage, termed EFDG1, was isolated from sewage effluents. The phage was visualized by electron microscopy. EFDG1 coding sequences and phylogeny were determined by whole genome sequencing (GenBank accession number KP339049), revealing it belongs to the Spounavirinae subfamily of the Myoviridae phages, which includes promising candidates for therapy against Gram-positive pathogens. This analysis also showed that the EFDG1 genome does not contain apparent harmful genes. EFDG1 antibacterial efficacy was evaluated in vitro against planktonic and biofilm cultures, showing effective lytic activity against various E. faecalis and E. faecium isolates, regardless of their antibiotic resistance profile. In addition, EFDG1 efficiently prevented ex vivo E. faecalis root canal infection. These findings suggest that phage therapy using EFDG1 might be efficacious to prevent E. faecalis infection after root canal treatment.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

