Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy
- PMID: 25663221
- PMCID: PMC4352097
- DOI: 10.1212/WNL.0000000000001334
Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy
Abstract
Objective: To report long-term efficacy and safety results of the SANTE trial investigating deep brain stimulation of the anterior nucleus of the thalamus (ANT) for treatment of localization-related epilepsy.
Methods: This long-term follow-up is a continuation of a previously reported trial of 5- vs 0-V ANT stimulation. Long-term follow-up began 13 months after device implantation with stimulation parameters adjusted at the investigators' discretion. Seizure frequency was determined using daily seizure diaries.
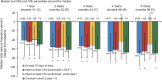
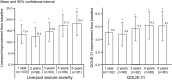
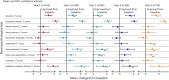
Results: The median percent seizure reduction from baseline at 1 year was 41%, and 69% at 5 years. The responder rate (≥50% reduction in seizure frequency) at 1 year was 43%, and 68% at 5 years. In the 5 years of follow-up, 16% of subjects were seizure-free for at least 6 months. There were no reported unanticipated adverse device effects or symptomatic intracranial hemorrhages. The Liverpool Seizure Severity Scale and 31-item Quality of Life in Epilepsy measure showed statistically significant improvement over baseline by 1 year and at 5 years (p < 0.001).
Conclusion: Long-term follow-up of ANT deep brain stimulation showed sustained efficacy and safety in a treatment-resistant population.
Classification of evidence: This long-term follow-up provides Class IV evidence that for patients with drug-resistant partial epilepsy, anterior thalamic stimulation is associated with a 69% reduction in seizure frequency and a 34% serious device-related adverse event rate at 5 years.
© 2015 American Academy of Neurology.
Figures


References
-
- Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia 2002;43:603–608. - PubMed
-
- Lee KJ, Jang KS, Shon YM. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir Suppl 2006;99:87–91. - PubMed
-
- Lim SN, Lee ST, Tsai YT, et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia 2007;48:342–347. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
