Amyloid and tau pathology of familial Alzheimer's disease APP/PS1 mouse model in a senescence phenotype background (SAMP8)
- PMID: 25663420
- PMCID: PMC4320125
- DOI: 10.1007/s11357-015-9747-3
Amyloid and tau pathology of familial Alzheimer's disease APP/PS1 mouse model in a senescence phenotype background (SAMP8)
Abstract
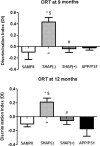
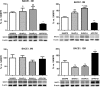
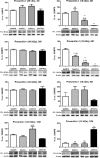
The amyloid precursor protein/presenilin 1 (APP/PS1) mouse model of Alzheimer's disease (AD) has provided robust neuropathological hallmarks of familial AD-like pattern at early ages, whereas senescence-accelerated mouse prone 8 (SAMP8) has a remarkable early senescence phenotype with pathological similarities to AD. The aim of this study was the investigation and characterization of cognitive and neuropathological AD markers in a novel mouse model that combines the characteristics of the APP/PS1 transgenic mouse model with a senescence-accelerated background of SAMP8 mice. Initially, significant differences were found regarding amyloid plaque formation and cognitive abnormalities. Bearing these facts in mind, we determined a general characterization of the main AD brain molecular markers, such as alterations in amyloid pathway, neuroinflammation, and hyperphosphorylation of tau in these mice along their lifetimes. Results from this analysis revealed that APP/PS1 in SAMP8 background mice showed alterations in the pathways studied in comparison with SAMP8 and APP/PS1, demonstrating that a senescence-accelerated background exacerbated the amyloid pathology and maintained the cognitive dysfunction present in APP/PS1 mice. Changes in tau pathology, including the activity of cyclin-dependent kinase 5 (CDK5) and glycogen synthase kinase 3 β (GSK3β), differs, but not in a parallel manner, with amyloid disturbances.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
