Formation and dissociation of the BSS1 protein complex regulates plant development via brassinosteroid signaling
- PMID: 25663622
- PMCID: PMC4456923
- DOI: 10.1105/tpc.114.131508
Formation and dissociation of the BSS1 protein complex regulates plant development via brassinosteroid signaling
Abstract
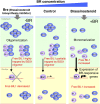
Brassinosteroids (BRs) play important roles in plant development and the response to environmental cues. BIL1/BZR1 is a master transcription factor in BR signaling, but the mechanisms that lead to the finely tuned targeting of BIL1/BZR1 by BRs are unknown. Here, we identified BRZ-SENSITIVE-SHORT HYPOCOTYL1 (BSS1) as a negative regulator of BR signaling in a chemical-biological analysis involving brassinazole (Brz), a specific BR biosynthesis inhibitor. The bss1-1D mutant, which overexpresses BSS1, exhibited a Brz-hypersensitive phenotype in hypocotyl elongation. BSS1 encodes a BTB-POZ domain protein with ankyrin repeats, known as BLADE ON PETIOLE1 (BOP1), which is an important regulator of leaf morphogenesis. The bss1-1D mutant exhibited an increased accumulation of phosphorylated BIL1/BZR1 and a negative regulation of BR-responsive genes. The number of fluorescent BSS1/BOP1-GFP puncta increased in response to Brz treatment, and the puncta were diffused by BR treatment in the root and hypocotyl. We show that BSS1/BOP1 directly interacts with BIL1/BZR1 or BES1. The large protein complex formed between BSS1/BOP1 and BIL1/BZR1 was only detected in the cytosol. The nuclear BIL1/BZR1 increased in the BSS1/BOP1-deficient background and decreased in the BSS1/BOP1-overexpressing background. Our study suggests that the BSS1/BOP1 protein complex inhibits the transport of BIL1/BZR1 to the nucleus from the cytosol and negatively regulates BR signaling.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures








Similar articles
-
BIL7 enhances plant growth by regulating the transcription factor BIL1/BZR1 during brassinosteroid signaling.Plant J. 2025 Jan;121(2):e17212. doi: 10.1111/tpj.17212. Epub 2024 Dec 20. Plant J. 2025. PMID: 39705127 Free PMC article.
-
Brassinosteroid-related transcription factor BIL1/BZR1 increases plant resistance to insect feeding.Biosci Biotechnol Biochem. 2014;78(6):960-8. doi: 10.1080/09168451.2014.910093. Epub 2014 Jun 26. Biosci Biotechnol Biochem. 2014. PMID: 25036120
-
Characterization of synthetic ecdysteroid analogues as functional mimics of brassinosteroids in plant growth.J Steroid Biochem Mol Biol. 2017 Sep;172:1-8. doi: 10.1016/j.jsbmb.2017.05.003. Epub 2017 May 4. J Steroid Biochem Mol Biol. 2017. PMID: 28479230
-
Molecular Mechanisms of Brassinosteroid-Mediated Responses to Changing Environments in Arabidopsis.Int J Mol Sci. 2020 Apr 15;21(8):2737. doi: 10.3390/ijms21082737. Int J Mol Sci. 2020. PMID: 32326491 Free PMC article. Review.
-
Interaction of the Transcription Factors BES1/BZR1 in Plant Growth and Stress Response.Int J Mol Sci. 2024 Jun 21;25(13):6836. doi: 10.3390/ijms25136836. Int J Mol Sci. 2024. PMID: 38999944 Free PMC article. Review.
Cited by
-
New Interacting Partners of BLADE-ON-PETIOLE in Regulation of Plant Development.Plant Physiol. 2019 Jun;180(2):697-698. doi: 10.1104/pp.19.00459. Plant Physiol. 2019. PMID: 31160524 Free PMC article. No abstract available.
-
Brassinosteroids regulate petal spur length in Aquilegia by controlling cell elongation.Ann Bot. 2021 Nov 9;128(7):931-942. doi: 10.1093/aob/mcab116. Ann Bot. 2021. PMID: 34508638 Free PMC article.
-
BLADE-ON-PETIOLE proteins act in an E3 ubiquitin ligase complex to regulate PHYTOCHROME INTERACTING FACTOR 4 abundance.Elife. 2017 Aug 22;6:e26759. doi: 10.7554/eLife.26759. Elife. 2017. PMID: 28826468 Free PMC article.
-
Pleiotropic effects of barley BLADE-ON-PETIOLE gene Uniculme4 on plant architecture and the jasmonic acid pathway.J Exp Bot. 2025 May 27;76(8):2177-2191. doi: 10.1093/jxb/eraf068. J Exp Bot. 2025. PMID: 40334057 Free PMC article.
-
BIL7 enhances plant growth by regulating the transcription factor BIL1/BZR1 during brassinosteroid signaling.Plant J. 2025 Jan;121(2):e17212. doi: 10.1111/tpj.17212. Epub 2024 Dec 20. Plant J. 2025. PMID: 39705127 Free PMC article.
References
-
- Arnaud N., Laufs P. (2013). Plant development: Brassinosteroids go out of bounds. Curr. Biol. 23: R152–R154. - PubMed
-
- Asami T., Mizutani M., Fujioka S., Goda H., Min Y.K., Shimada Y., Nakano T., Takatsuto S., Matsuyama T., Nagata N., Sakata K., Yoshida S. (2001). Selective interaction of triazole derivatives with DWF4, a cytochrome P450 monooxygenase of the brassinosteroid biosynthetic pathway, correlates with brassinosteroid deficiency in planta. J. Biol. Chem. 276: 25687–25691. - PubMed
-
- Asami T., Nakano T., Nakashita H., Sekimata K., Shimada Y., Yoshida S. (2003). The influence of chemical genetics on plant science: Shedding light on functions and mechanism of action of brassinosteroids using biosynthesis inhibitors. J. Plant Growth Regul. 22: 336–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

