The biochemistry of mitosis
- PMID: 25663668
- PMCID: PMC4355272
- DOI: 10.1101/cshperspect.a015776
The biochemistry of mitosis
Abstract
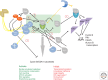
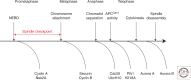
In this article, we will discuss the biochemistry of mitosis in eukaryotic cells. We will focus on conserved principles that, importantly, are adapted to the biology of the organism. It is vital to bear in mind that the structural requirements for division in a rapidly dividing syncytial Drosophila embryo, for example, are markedly different from those in a unicellular yeast cell. Nevertheless, division in both systems is driven by conserved modules of antagonistic protein kinases and phosphatases, underpinned by ubiquitin-mediated proteolysis, which create molecular switches to drive each stage of division forward. These conserved control modules combine with the self-organizing properties of the subcellular architecture to meet the specific needs of the cell. Our discussion will draw on discoveries in several model systems that have been important in the long history of research on mitosis, and we will try to point out those principles that appear to apply to all cells, compared with those in which the biochemistry has been specifically adapted in a particular organism.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Amon A, Surana U, Muroff I, Nasmyth K 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355: 368–371. - PubMed
-
- Bayliss R, Sardon T, Vernos I, Conti E 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell 12: 851–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
