Integrin traffic - the update
- PMID: 25663697
- PMCID: PMC4342575
- DOI: 10.1242/jcs.161653
Integrin traffic - the update
Abstract
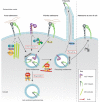
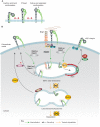
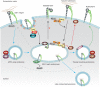
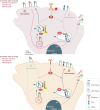
Integrins are a family of transmembrane cell surface molecules that constitute the principal adhesion receptors for the extracellular matrix (ECM) and are indispensable for the existence of multicellular organisms. In vertebrates, 24 different integrin heterodimers exist with differing substrate specificity and tissue expression. Integrin-extracellular-ligand interaction provides a physical anchor for the cell and triggers a vast array of intracellular signalling events that determine cell fate. Dynamic remodelling of adhesions, through rapid endocytic and exocytic trafficking of integrin receptors, is an important mechanism employed by cells to regulate integrin-ECM interactions, and thus cellular signalling, during processes such as cell migration, invasion and cytokinesis. The initial concept of integrin traffic as a means to translocate adhesion receptors within the cell has now been expanded with the growing appreciation that traffic is intimately linked to the cell signalling apparatus. Furthermore, endosomal pathways are emerging as crucial regulators of integrin stability and expression in cells. Thus, integrin traffic is relevant in a number of pathological conditions, especially in cancer. Nearly a decade ago we wrote a Commentary in Journal of Cell Science entitled 'Integrin traffic'. With the advances in the field, we felt it would be appropriate to provide the growing number of researchers interested in integrin traffic with an update.
Keywords: Integrin; Invasion; Migration; Rab GTPases; Signalling crosstalk; Trafficking.
© 2015. Published by The Company of Biologists Ltd.
Figures
References
-
- Bates R. C., Bellovin D. I., Brown C., Maynard E., Wu B., Kawakatsu H., Sheppard D., Oettgen P., Mercurio A. M. (2005). Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 115, 339–347 10.1172/JCI200523183 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

