Swept confocally-aligned planar excitation (SCAPE) microscopy for high speed volumetric imaging of behaving organisms
- PMID: 25663846
- PMCID: PMC4317333
- DOI: 10.1038/nphoton.2014.323
Swept confocally-aligned planar excitation (SCAPE) microscopy for high speed volumetric imaging of behaving organisms
Abstract
We report a new 3D microscopy technique that allows volumetric imaging of living samples at ultra-high speeds: Swept, confocally-aligned planar excitation (SCAPE) microscopy. While confocal and two-photon microscopy have revolutionized biomedical research, current implementations are costly, complex and limited in their ability to image 3D volumes at high speeds. Light-sheet microscopy techniques using two-objective, orthogonal illumination and detection require a highly constrained sample geometry, and either physical sample translation or complex synchronization of illumination and detection planes. In contrast, SCAPE microscopy acquires images using an angled, swept light-sheet in a single-objective, en-face geometry. Unique confocal descanning and image rotation optics map this moving plane onto a stationary high-speed camera, permitting completely translationless 3D imaging of intact samples at rates exceeding 20 volumes per second. We demonstrate SCAPE microscopy by imaging spontaneous neuronal firing in the intact brain of awake behaving mice, as well as freely moving transgenic Drosophila larvae.
Figures
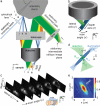




References
-
- Dodt H-U, et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nature Methods. 2007;4:331–336. - PubMed
-
- Verveer PJ, et al. High-resolution three-dimensional imaging of large specimens with light sheet-based microscopy. Nat Methods. 2007;4:311–313. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
