Strain amplification analysis of an osteocyte under static and cyclic loading: a finite element study
- PMID: 25664319
- PMCID: PMC4312579
- DOI: 10.1155/2015/376474
Strain amplification analysis of an osteocyte under static and cyclic loading: a finite element study
Abstract
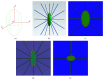
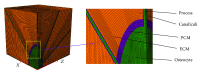
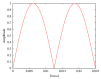
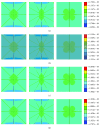
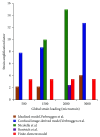
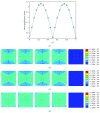
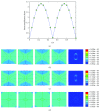
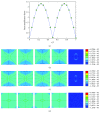
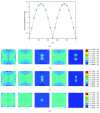
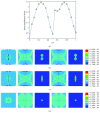
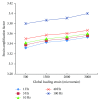
Osteocytes, the major type of bone cells which reside in their lacunar and canalicular system within the bone matrix, function as biomechanosensors and biomechanotransducers of the bone. Although biomechanical behaviour of the osteocyte-lacunar-canalicular system has been investigated in previous studies mostly using computational 2-dimensional (2D) geometric models, only a few studies have used the 3-dimensional (3D) finite element (FE) model. In the current study, a 3D FE model was used to predict the responses of strain distributions of osteocyte-lacunar-canalicular system analyzed under static and cyclic loads. The strain amplification factor was calculated for all simulations. Effects on the strain of the osteocyte system were investigated under 500, 1500, 2000, and 3000 microstrain loading magnitudes and 1, 5, 10, 40, and 100 Hz loading frequencies. The maximum strain was found to change with loading magnitude and frequency. It was observed that maximum strain under 3000-microstrain loading was higher than those under 500, 1500, and 2000 microstrains. When the loading strain reached the maximum magnitude, the strain amplification factor of 100 Hz was higher than those of the other frequencies. Data from this 3D FE model study suggests that the strain amplification factor of the osteocyte-lacunar-canalicular system increases with loading frequency and loading strain increasing.
Figures

Similar articles
-
Synchrotron X-ray phase nano-tomography-based analysis of the lacunar-canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis.Biomech Model Mechanobiol. 2015 Apr;14(2):267-82. doi: 10.1007/s10237-014-0601-9. Epub 2014 Jul 11. Biomech Model Mechanobiol. 2015. PMID: 25011566
-
Osteocyte lacunar strain determination using multiscale finite element analysis.Bone Rep. 2020 May 19;12:100277. doi: 10.1016/j.bonr.2020.100277. eCollection 2020 Jun. Bone Rep. 2020. PMID: 32478144 Free PMC article.
-
Computational Investigation on the Biomechanical Responses of the Osteocytes to the Compressive Stimulus: A Poroelastic Model.Biomed Res Int. 2018 Jan 18;2018:4071356. doi: 10.1155/2018/4071356. eCollection 2018. Biomed Res Int. 2018. PMID: 29581973 Free PMC article.
-
Measurement and estimation of osteocyte mechanical strain.Bone. 2013 Jun;54(2):191-5. doi: 10.1016/j.bone.2013.01.037. Epub 2013 Jan 28. Bone. 2013. PMID: 23369990 Review.
-
Influence of Osteocyte Lacunar-Canalicular Morphology and Network Architecture on Osteocyte Mechanosensitivity.Curr Osteoporos Rep. 2023 Aug;21(4):401-413. doi: 10.1007/s11914-023-00792-9. Epub 2023 Jun 5. Curr Osteoporos Rep. 2023. PMID: 37273086 Review.
Cited by
-
3D-Bioprinting Strategies Based on In Situ Bone-Healing Mechanism for Vascularized Bone Tissue Engineering.Micromachines (Basel). 2021 Mar 8;12(3):287. doi: 10.3390/mi12030287. Micromachines (Basel). 2021. PMID: 33800485 Free PMC article. Review.
-
The effect of tail suspension and treadmill exercise on LRP6 expression, bone mass and biomechanical properties of hindlimb bones in SD rats.Am J Transl Res. 2019 Sep 15;11(9):5847-5857. eCollection 2019. Am J Transl Res. 2019. PMID: 31632553 Free PMC article.
-
Comparison of strain measurement in the mouse forearm using subject-specific finite element models, strain gaging, and digital image correlation.Biomech Model Mechanobiol. 2017 Aug;16(4):1243-1253. doi: 10.1007/s10237-017-0885-7. Epub 2017 Feb 15. Biomech Model Mechanobiol. 2017. PMID: 28204985 Free PMC article.
-
Finite Element Models of Osteocytes and Their Load-Induced Activation.Curr Osteoporos Rep. 2022 Apr;20(2):127-140. doi: 10.1007/s11914-022-00728-9. Epub 2022 Mar 17. Curr Osteoporos Rep. 2022. PMID: 35298773 Free PMC article. Review.
-
Mechanical Stimuli in the Local In Vivo Environment in Bone: Computational Approaches Linking Organ-Scale Loads to Cellular Signals.Curr Osteoporos Rep. 2018 Aug;16(4):395-403. doi: 10.1007/s11914-018-0448-6. Curr Osteoporos Rep. 2018. PMID: 29915967 Free PMC article. Review.
References
-
- You L.-D., Weinbaum S., Cowin S. C., Schaffler M. B. Ultrastructure of the osteocyte process and its pericellular matrix. Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2004;278(2):505–513. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

