Hydraulic fracture during epithelial stretching
- PMID: 25664452
- PMCID: PMC4374166
- DOI: 10.1038/nmat4206
Hydraulic fracture during epithelial stretching
Abstract
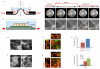
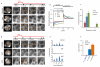
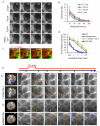
The origin of fracture in epithelial cell sheets subject to stretch is commonly attributed to excess tension in the cells' cytoskeleton, in the plasma membrane, or in cell-cell contacts. Here, we demonstrate that for a variety of synthetic and physiological hydrogel substrates the formation of epithelial cracks is caused by tissue stretching independently of epithelial tension. We show that the origin of the cracks is hydraulic; they result from a transient pressure build-up in the substrate during stretch and compression manoeuvres. After pressure equilibration, cracks heal readily through actomyosin-dependent mechanisms. The observed phenomenology is captured by the theory of poroelasticity, which predicts the size and healing dynamics of epithelial cracks as a function of the stiffness, geometry and composition of the hydrogel substrate. Our findings demonstrate that epithelial integrity is determined in a tension-independent manner by the coupling between tissue stretching and matrix hydraulics.
Figures


Comment in
-
Cell mechanics: Hydraulic cracking.Nat Mater. 2015 Mar;14(3):268-9. doi: 10.1038/nmat4229. Nat Mater. 2015. PMID: 25698432 No abstract available.
References
-
- Alberts B. Molecular Biology of the cell. 2002 Garland Science.
-
- Fung YC. Biomechanics: mechanical properties of living tissues. 1993
-
- Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–1189. - PubMed
-
- Bosveld F, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–727. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

