Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis
- PMID: 25664595
- PMCID: PMC5024041
- DOI: 10.1111/ene.12664
Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis
Erratum in
-
Corrigendum.Eur J Neurol. 2017 Apr;24(4):659. doi: 10.1111/ene.13282. Eur J Neurol. 2017. PMID: 28322002 Free PMC article. No abstract available.
Abstract
Background and purpose: Tauroursodeoxycholic acid (TUDCA) is a hydrophilic bile acid that is produced in the liver and used for treatment of chronic cholestatic liver diseases. Experimental studies suggest that TUDCA may have cytoprotective and anti-apoptotic action, with potential neuroprotective activity. A proof of principle approach was adopted to provide preliminary data regarding the efficacy and tolerability of TUDCA in a series of patients with amyotrophic lateral sclerosis (ALS).
Methods: As a proof of principle, using a double-blind placebo controlled design, 34 ALS patients under treatment with riluzole who were randomized to placebo or TUDCA (1 g twice daily for 54 weeks) were evaluated after a lead-in period of 3 months. The patients were examined every 6 weeks. The primary outcome was the proportion of responders [those subjects with improvement of at least 15% in the Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) slope during the treatment period compared to the lead-in phase]. Secondary outcomes included between-treatment comparison of ALSFRS-R at study end, comparison of the linear regression slopes for ALSFFRS-R mean scores and the occurrence of adverse events.
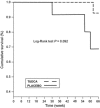
Results: Tauroursodeoxycholic acid was well tolerated; there were no between-group differences for adverse events. The proportion of responders was higher under TUDCA (87%) than under placebo (P = 0.021; 43%). At study end baseline-adjusted ALSFRS-R was significantly higher (P = 0.007) in TUDCA than in placebo groups. Comparison of the slopes of regression analysis showed slower progression in the TUDCA than in the placebo group (P < 0.01).
Conclusions: This pilot study provides preliminary clinical data indicating that TUDCA is safe and may be effective in ALS.
Trial registration: ClinicalTrials.gov NCT00877604.
Keywords: amyotrophic lateral sclerosis; cholic acids; tauroursodeoxycholic acid.
© 2015 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Figures

Comment in
-
The TUDCA trial--innovative trial designs for amyotrophic lateral sclerosis drugs?Eur J Neurol. 2016 Jan;23(1):11-2. doi: 10.1111/ene.12691. Epub 2015 Apr 1. Eur J Neurol. 2016. PMID: 25828295 No abstract available.
References
-
- Ince PG, Lowe J, Shaw PJ. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol 1998; 24: 104–117. - PubMed
-
- Cozzolino M, Ferri A, Valle C, Carri MT. Mitochondria and ALS: implications from novel genes and pathways. Mol Cell Neurosci 2013; 55: 44–49. - PubMed
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose‐ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996; 347: 1425–1431. - PubMed
-
- Sreedharan J, Brown RH Jr. Amyotrophic lateral sclerosis: problems and prospects. Ann Neurol 2013; 74: 309–316. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

