Hyperoxia downregulates angiotensin-converting enzyme-2 in human fetal lung fibroblasts
- PMID: 25665060
- PMCID: PMC5119454
- DOI: 10.1038/pr.2015.27
Hyperoxia downregulates angiotensin-converting enzyme-2 in human fetal lung fibroblasts
Abstract
Background: Angiotensin (ANG) II is involved in experimental hyperoxia-induced lung fibrosis. Angiotensin-converting enzyme-2 (ACE-2) degrades ANG II and is thus protective, but is downregulated in adult human and experimental lung fibrosis. Hyperoxia is a known cause of chronic fibrotic lung disease in neonates, but the role of ACE-2 in neonatal lung fibrosis is unknown. We hypothesized that ACE-2 in human fetal lung cells might be downregulated by hyperoxic gas.
Methods: Fetal human lung fibroblast IMR90 cells were exposed to hyperoxic (95% O2/5% CO2) or normoxic (21% O2/5% CO2) gas in vitro. Cells and culture media were recovered separately for assays of ACE-2 enzymatic activity, mRNA, and immunoreactive protein.
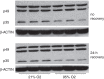
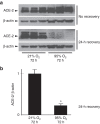
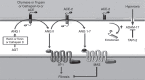
Results: Hyperoxia decreased ACE-2 immunoreactive protein and enzyme activity in IMR90 cells (both P < 0.01), but did not change ACE-2 mRNA. ACE-2 protein was increased in the cell supernatant, suggesting protease-mediated ectodomain shedding. TAPI-2, an inhibitor of TNF-α-converting enzyme (TACE/ADAM17), prevented both the decrease in cellular ACE-2 and the increase in soluble ACE-2 (both P < 0.05).
Conclusion: These data show that ACE-2 is expressed in fetal human lung fibroblasts but is significantly decreased by hyperoxic gas. They also suggest that hyperoxia decreases ACE-2 through a shedding mechanism mediated by ADAM17/TACE.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

