Regulation of mammalian Ste20 (Mst) kinases
- PMID: 25665457
- PMCID: PMC4340714
- DOI: 10.1016/j.tibs.2015.01.001
Regulation of mammalian Ste20 (Mst) kinases
Abstract
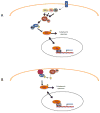
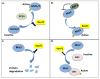
Initially identified as mammalian homologs to yeast Ste20 kinases, the mammalian sterile twenty-like (Mst) 1/2 kinases have been widely investigated subsequent to their rediscovery as key components of the Hippo tumor suppressor pathway in flies. To date, our understanding of Mst substrates and downstream signaling outstrips our knowledge of how these enzymes are controlled by upstream signals. While much remains to be discovered regarding the mechanisms of Mst regulation, it is clear that Mst1 kinase activity is governed at least in part by its state of dimerization, including self-association and also heterodimerization with various other signaling partners. Here we review the basic architecture of Mst signaling and function and discuss recent advances in our understanding of how these important kinases are regulated.
Keywords: dimerization; serine/threonine protein kinases; signal transduction; tumor suppressor.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Biosignaling of mammalian Ste20-related kinases.Cell Signal. 2008 Jul;20(7):1237-47. doi: 10.1016/j.cellsig.2007.12.019. Epub 2008 Jan 4. Cell Signal. 2008. PMID: 18255267 Review.
-
Cloning and characterization of a human STE20-like protein kinase with unusual cofactor requirements.J Biol Chem. 1997 Nov 7;272(45):28695-703. doi: 10.1074/jbc.272.45.28695. J Biol Chem. 1997. PMID: 9353338
-
The DeMSTification of mammalian Ste20 kinases.Curr Biol. 2009 May 26;19(10):R421-5. doi: 10.1016/j.cub.2009.04.022. Curr Biol. 2009. PMID: 19467213 Review.
-
The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation.Curr Biol. 2009 Nov 3;19(20):1692-702. doi: 10.1016/j.cub.2009.09.020. Curr Biol. 2009. PMID: 19836237
-
Mechanisms of Hippo pathway regulation.Genes Dev. 2016 Jan 1;30(1):1-17. doi: 10.1101/gad.274027.115. Genes Dev. 2016. PMID: 26728553 Free PMC article. Review.
Cited by
-
SARAH Domain-Mediated MST2-RASSF Dimeric Interactions.PLoS Comput Biol. 2016 Oct 7;12(10):e1005051. doi: 10.1371/journal.pcbi.1005051. eCollection 2016 Oct. PLoS Comput Biol. 2016. PMID: 27716844 Free PMC article.
-
Biochemical purification uncovers mammalian sterile 3 (MST3) as a new protein kinase for multifunctional protein kinases AMPK and SIK3.J Biol Chem. 2022 May;298(5):101929. doi: 10.1016/j.jbc.2022.101929. Epub 2022 Apr 10. J Biol Chem. 2022. PMID: 35413286 Free PMC article.
-
Tumor suppressor C-RASSF proteins.Cell Mol Life Sci. 2018 May;75(10):1773-1787. doi: 10.1007/s00018-018-2756-5. Epub 2018 Jan 20. Cell Mol Life Sci. 2018. PMID: 29353317 Free PMC article. Review.
-
Immune-associated proteins with potential in vivo anti-tumor activities are upregulated in lung cancer cells treated with umbelliprenin: A proteomic approach.Oncol Lett. 2016 Dec;12(6):5295-5302. doi: 10.3892/ol.2016.5352. Epub 2016 Nov 4. Oncol Lett. 2016. PMID: 28105238 Free PMC article.
-
Hras helps hippo heterodimerize to evade tumor suppression.Small GTPases. 2018 Jul 4;9(4):327-331. doi: 10.1080/21541248.2016.1228794. Epub 2016 Sep 20. Small GTPases. 2018. PMID: 27560370 Free PMC article. Review.
References
-
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer cell. 2008;13:188–192. - PubMed
-
- Creasy CL, et al. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. The Journal of biological chemistry. 1996;271:21049–21053. - PubMed
-
- Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

