Regulation of mammalian Ste20 (Mst) kinases
- PMID: 25665457
- PMCID: PMC4340714
- DOI: 10.1016/j.tibs.2015.01.001
Regulation of mammalian Ste20 (Mst) kinases
Abstract
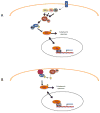
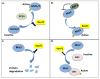
Initially identified as mammalian homologs to yeast Ste20 kinases, the mammalian sterile twenty-like (Mst) 1/2 kinases have been widely investigated subsequent to their rediscovery as key components of the Hippo tumor suppressor pathway in flies. To date, our understanding of Mst substrates and downstream signaling outstrips our knowledge of how these enzymes are controlled by upstream signals. While much remains to be discovered regarding the mechanisms of Mst regulation, it is clear that Mst1 kinase activity is governed at least in part by its state of dimerization, including self-association and also heterodimerization with various other signaling partners. Here we review the basic architecture of Mst signaling and function and discuss recent advances in our understanding of how these important kinases are regulated.
Keywords: dimerization; serine/threonine protein kinases; signal transduction; tumor suppressor.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer cell. 2008;13:188–192. - PubMed
-
- Creasy CL, et al. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. The Journal of biological chemistry. 1996;271:21049–21053. - PubMed
-
- Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

