A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: application to small vertebrate lens imaging
- PMID: 25665708
- PMCID: PMC4379205
- DOI: 10.1007/s00216-015-8489-5
A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: application to small vertebrate lens imaging
Abstract
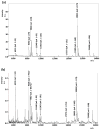
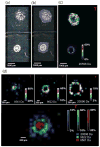
MALDI imaging requires careful sample preparation to obtain reliable, high-quality images of small molecules, peptides, lipids, and proteins across tissue sections. Poor crystal formation, delocalization of analytes, and inadequate tissue adherence can affect the quality, reliability, and spatial resolution of MALDI images. We report a comparison of tissue mounting and washing methods that resulted in an optimized method using conductive carbon substrates that avoids thaw mounting or washing steps, minimizes protein delocalization, and prevents tissue detachment from the target surface. Application of this method to image ocular lens proteins of small vertebrate eyes demonstrates the improved methodology for imaging abundant crystallin protein products. This method was demonstrated for tissue sections from rat, mouse, and zebrafish lenses resulting in good-quality MALDI images with little to no delocalization. The images indicate, for the first time in mouse and zebrafish, discrete localization of crystallin protein degradation products resulting in concentric rings of distinct protein contents that may be responsible for the refractive index gradient of vertebrate lenses.
Figures




References
-
- Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: location of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–4760. - PubMed
-
- Stoeckli M, Staab D, Schweitzer A. Compound and metabolite distribution measured by MALDI mass spectrometry imaging in whole body tissue sections. Int J Mass Spectrom. 2007;2:195–202.
-
- Anderson DMG, Carolan VA, Crosland S, Sharples KR, Clench MR. Examination of the translocation of sulfonylurea herbicides in sunflower plants by matrix-assisted laser desorption/ionisation mass spectrometry imaging. Rapid Comm in Mass Spec. 2010;24:3309–3319. - PubMed
-
- Castellino S, Groseclose MR, Wagner D. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development. Bioanalysis. 2011;3:2427–41. - PubMed
-
- Trim PJ, Henson CM, Avery JL, McEwen A, Snel MF, Claude E, Marshall PS, West A, Princivalle AP, Clench MR. Matrix-assisted laser desorption/ionization-ion mobility separation-mass spectrometry imaging of vinblastine in whole body tissue sections. Anal Chem. 2008;80:8628–8634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

