Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice
- PMID: 25665752
- PMCID: PMC4511166
- DOI: 10.1016/j.neuroscience.2015.01.068
Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice
Abstract
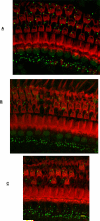
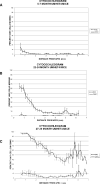
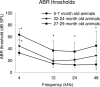
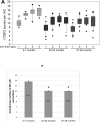
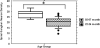
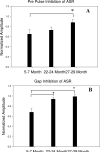
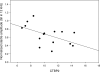
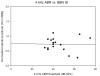
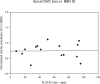
This study compared the timing of appearance of three components of age-related hearing loss that determine the pattern and severity of presbycusis: the functional and structural pathologies of sensory cells and neurons and changes in gap detection (GD), the latter as an indicator of auditory temporal processing. Using UM-HET4 mice, genetically heterogeneous mice derived from four inbred strains, we studied the integrity of inner and outer hair cells by position along the cochlear spiral, inner hair cell-auditory nerve connections, spiral ganglion neurons (SGN), and determined auditory thresholds, as well as pre-pulse and gap inhibition of the acoustic startle reflex (ASR). Comparisons were made between mice of 5-7, 22-24 and 27-29 months of age. There was individual variability among mice in the onset and extent of age-related auditory pathology. At 22-24 months of age a moderate to large loss of outer hair cells was restricted to the apical third of the cochlea and threshold shifts in the auditory brain stem response were minimal. There was also a large and significant loss of inner hair cell-auditory nerve connections and a significant reduction in GD. The expression of Ntf3 in the cochlea was significantly reduced. At 27-29 months of age there was no further change in the mean number of synaptic connections per inner hair cell or in GD, but a moderate to large loss of outer hair cells was found across all cochlear turns as well as significantly increased ABR threshold shifts at 4, 12, 24 and 48 kHz. A statistical analysis of correlations on an individual animal basis revealed that neither the hair cell loss nor the ABR threshold shifts correlated with loss of GD or with the loss of connections, consistent with independent pathological mechanisms.
Keywords: NT3; age-related hearing loss; aging; auditory; cochlea; gap detection.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
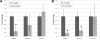
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

