Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines
- PMID: 25665925
- PMCID: PMC5389140
- DOI: 10.1038/srep08220
Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines
Abstract
The opportunistic fungal pathogen Aspergillus fumigatus is increasingly found as a coinfecting agent along with Pseudomonas aeruginosa in cystic fibrosis patients. Amongst the numerous molecules secreted by P. aeruginosa during its growth, phenazines constitute a major class. P. aeruginosa usually secreted four phenazines, pyocyanin (PYO), phenazine-1-carboxamide (PCN), 1-hydroxyphenazine (1-HP) and phenazine-1-carboxylic acid (PCA). These phenazines inhibited the growth of A. fumigatus but the underlying mechanisms and the impact of these four phenazines on A. fumigatus biology were not known. In the present study, we analyzed the functions of the four phenazines and their mode of action on A. fumigatus. All four phenazines showed A. fumigatus growth inhibitory effects by inducing production of reactive oxygen species (ROS), specifically O2(·-), and reactive nitrogen species (RNS), ONOO(-). A. fumigatus Sod2p was the major factor involved in resistance against the ROS and RNS induced by phenazines. Sub-inhibitory concentrations of PYO, PCA and PCN promote A. fumigatus growth by an independent iron-uptake acquisition. Of the four phenazines 1-HP had a redox-independent function; being able to chelate metal ions 1-HP induced A. fumigatus iron starvation. Our data show the fine-interactions existing between A. fumigatus and P. aeruginosa, which can lead to stimulatory or antagonistic effects.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
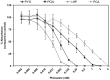








 ) ΔsidA and (
) ΔsidA and ( ) ΔsidAΔftrA mutants incubated in presence of increased concentration of phenazines. (– –) ΔsidA incubated in absence of phenazines. The growth was quantified at absorbance 560 nm following the crystal violet procedure.
) ΔsidAΔftrA mutants incubated in presence of increased concentration of phenazines. (– –) ΔsidA incubated in absence of phenazines. The growth was quantified at absorbance 560 nm following the crystal violet procedure.
 ). Phenazines act at the level of mitochondria and induce the production of superoxide anion (O2·−) and peroxynitrite (ONOO−) (Red pathway,
). Phenazines act at the level of mitochondria and induce the production of superoxide anion (O2·−) and peroxynitrite (ONOO−) (Red pathway, ). MnSod2p enzyme converts O2·− to hydrogen peroxide (H2O2) and a catalase-independent mechanism allows its detoxification into H2O. The anion ONOO− formed from O2·− and nitric oxide (NO·) is also detoxified by MnSod2p (Red pathway,
). MnSod2p enzyme converts O2·− to hydrogen peroxide (H2O2) and a catalase-independent mechanism allows its detoxification into H2O. The anion ONOO− formed from O2·− and nitric oxide (NO·) is also detoxified by MnSod2p (Red pathway, ). PYO, PCN and PCA reduce Fe(III) to Fe(II) which penetrates the A. fumigatus cell through the iron ferroxidase FetCp/permease FtrAp complex (Green pathway,
). PYO, PCN and PCA reduce Fe(III) to Fe(II) which penetrates the A. fumigatus cell through the iron ferroxidase FetCp/permease FtrAp complex (Green pathway, ). 1-HP also reduces Fe(III) to Fe(II) and two 1-HP molecules can chelate the newly formed Fe(II) (Orange pathway,
). 1-HP also reduces Fe(III) to Fe(II) and two 1-HP molecules can chelate the newly formed Fe(II) (Orange pathway, ). This chelating activity induces iron starvation which causes HapXp activation. The biosynthetic pathway of triacetylfusarinine C (TAFC) is then activated and allows Fe3+ acquisition to stimulate the growth of the fungus (Orange pathway,
). This chelating activity induces iron starvation which causes HapXp activation. The biosynthetic pathway of triacetylfusarinine C (TAFC) is then activated and allows Fe3+ acquisition to stimulate the growth of the fungus (Orange pathway, ).
).References
-
- Price-Whelan A., Dietrich L. E. P. & Newman D. K. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2, 71–78 (2006). - PubMed
-
- Lau G. W., Hassett D. J., Ran H. & Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 10, 599–606 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

